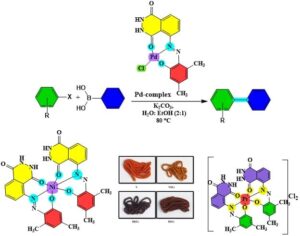
A novel ligand, (E)-5-((2-hydroxy-4,6-dimethylphenyl)diazenyl)-2,3-dihydrophthalazine-1,4-dione, was synthesized through the reaction of 3,5-dimethylphenol with the diazonium salt of 5-amino-2,3-dihydrophthalazine-1,4-dione. The ligand underwent characterization through the utilization of diverse spectroscopic methods, including UV-Vis, FT-IR, 13C and 1H-NMR, alongside Mass spectroscopy and microelemental analysis (Carbon, Hydrogen, Nitrogen, Oxygen). Metal chelates of transition metals were prepared and analyzed using […]
Cu-catalysis was employed to create numerous (E)-5-bromo-N-aryl oxindole nitrones with satisfactory to excellent coupling efficiencies. This process involved the reaction of N-unprotected 5-bromoisatin-3-oximes and aryl-boronic acid derivatives under mild reactive conditions. Remarkably, the reaction exhibited tolerance towards several aryl-boronic acids containing diverse functional sensitivity subgroups (73% to 97% yields and 2 to 6 h). Extensive […]
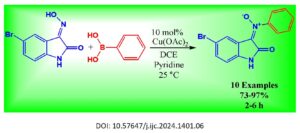
Cu-catalysis was employed to create numerous (E)-5-bromo-N-aryl oxindole nitrones with satisfactory to excellent coupling efficiencies. This process involved the reaction of N-unprotected 5-bromoisatin-3-oximes and aryl-boronic acid derivatives under mild reactive conditions. Remarkably, the reaction exhibited tolerance towards several aryl-boronic acids containing diverse functional sensitivity subgroups (73% to 97% yields and 2 to 6 h). Extensive […]
Sulfuric acid ([3-(3-silicapropyl)sulfanyl]propyl]ester (SASPSPE) is employed as a recyclable catalyst for the silylation of hydroxyl groups. A good range of primary, secondary alcohols and phenolic hydroxyl groups were effectively converted into their corresponding trimethylsilyl ethers with hexamethyldisilazane in the presence of catalytic amounts of SASPSPE at room temperature with short reaction times in good to […]
A simple and mild method for tetrahydropyranylation of alcohols is reported at room temperature using a catalytic amount of 3-methylimidazolinium hydrogensulfate([Hmim]HSO4) as a green, efficient and reusable catalyst. Using solvent-free conditions, non-toxic and inexpensive materials, modest and clean work-up, short reaction times and high yields of the products are the advantages of this method.
The one-pot, multi-component synthesis of 4,4′-(arylmethylene)bis(1H-pyrazol-5-ols) by tandem Knoevenagel-Michael reaction of phenylhydrazine, ethyl acetoacetate and aldehydes in the presence of silica-bonded N-propylpiperazine sulfamic acid (SBPPSA) as a recyclable solid acid catalyst was reported. SBPPSA showed much the same efficiency when used in consecutive reaction runs.
The application of the [Pd{C6H3(CH2CH2NH2)-4-OMe-5-κ2-C,N}(μ-Br)]2 complex of 2-methoxyphenethylamine in the Heck coupling reaction was considered under both conventional and microwave irradiation conditions, and their results were compared. This complex is an efficient, stable and non-sensitive to air and moisture catalyst for the vinylation of substituted aryl halides with different electronic properties. The cross-coupled products were […]
A highly practical and efficient preparation of pyrano[3,2-c]pyridone and pyrano[3,2-c]quinoline derivatives was developed via an ionic liquid mediated and promoted multi-component reaction of malononitrile, aldehyde, and 4-hydroxyquinolin-2(1H)-one or 4-hydroxy-6-methylpyridin-2(1H)-one. The combinatorial syntheses were achieved for the first time without applying extra activation energy at ambient temperature while making use of [BMIm]Cl as a catalyst solvent.
Silica gel has been used for synthesis of biscoumarin derivatives starting from 4-hydroxycoumarin and aryl aldehydes. The reaction conditions are completely in agreement with green chemistry principles including sing water as media and a recyclable and safe catalyst. This method is also simple and inexpensive and leads to high yield of products in short reaction […]
Sulfuric acid {[3-(3-silicapropyl)sulfanyl]propyl}ester (SASPSPE) is employed as a recyclable catalyst for the synthesis of α-amino nitriles. These syntheses were performed via a one-pot three-component condensation of aldehydes, amines, and trimethylsilyl cyanide under mild reaction conditions at room temperature. The catalyst could be recycled and reused several times without any loss of efficiency.