Original Article
Cascades Synthesis of N-Aryl Oxindole Nitrone Derivatives using Isatine Oximes and Arylboronic acids Catalyzed by Copper Acetate under Mild Conditions
Authors
-
Rita S. Adam
*
 1
1
Abstract
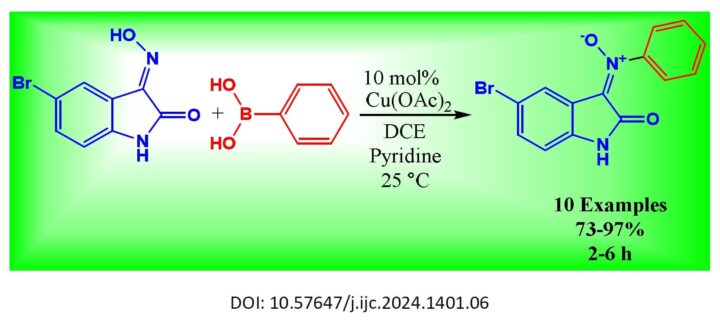
Cu-catalysis was employed to create numerous (E)-5-bromo-N-aryl oxindole nitrones with satisfactory to excellent coupling efficiencies. This process involved the reaction of N-unprotected 5-bromoisatin-3-oximes and aryl-boronic acid derivatives under mild reactive conditions. Remarkably, the reaction exhibited tolerance towards several aryl-boronic acids containing diverse functional sensitivity subgroups (73% to 97% yields and 2 to 6 h). Extensive research has demonstrated that the (C=O) group present in 5-bromoisatin-3-oximes can serve as an inhibitory molecule or ligand, playing a crucial role in the generation of (E) oxindole nitrones.
Highlights:
- In this research copper catalyzed cabon-nitrogen bond formatio
- 10 derivatives of N-aryl oxindole nitrone were prepared
- The starting materials were different aryl boronic acid and N-unprotected 5-bromoisatin-3-oximes\A reasonable mechanism was suggested