Synthesis of Fe-doped TiO2 with improved photocatalytic properties under Vis-L irradiation
Authors
-
Imane Ellouzi
*
 1
1
- Boutaina Regraguy 1
- Souad El Hajjaji 1
- Mourad Harir 2
- Philippe Schmitt-Kopplin 3
-
Hinda Lachheb
 2
2
- Larbi Laânab 3
Abstract
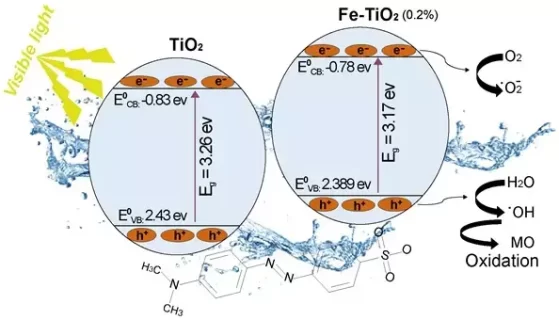
Fe-doped TiO2 nanoparticles were successfully synthesized by the coprecipitation method. TiO2 was doped with a different molar ratio of iron amounts, namely 0.1% and 0.2%. An undoped TiO2 was also prepared for comparison. X-ray diffraction (XRD), transmission electron microscopy (TEM) and UV-visible diffuse reflectance spectroscopy techniques were used to characterize the as-synthesized nanoparticles. The XRD spectra revealed that the photocatalysts were mostly in a well-crystallized anatase phase. Optical properties of the powders shifted from UV to the beginning of the visible light (Vis-L) region. Absorption edge wavelengths between 392 and 380 nm were obtained for the Fe-doped TiO2 and TiO2-P25, corresponding to band gap energies between 3.17 and 3.26 eV. TEM images showed homogeneity with a certain degree of agglomeration for all the samples. The photocatalytic efficiency of the as-synthesized Fe-doped TiO2 nanoparticles was performed using azo dye methyl orange (MO) in an aqueous solution under Vis-L irradiation. The photocatalytic results showed that Fe-doped TiO2 nanoparticles effectively degrade MO under Vis-L excitation and follow pseudo-first order kinetics. Besides, kinetic comparison showed that pure TiO2 is less efficient than 0.1% and 0.2% Fe-doped TiO2 because they exhibit unequaled efficiency. Moreover, the photocatalyst at 0.2% Fe-doped TiO2 molar ratio revealed the highest photocatalytic efficiency, which was 4.2 times higher compared to pure TiO2. Different amounts of Fe induced different increases in the apparent first-order rate constant of the photocatalytic process.
Highlights
- Synthesis of Fe/TiO2 via coprecipitation method.
- Fe-TiO2 materials observed greater performance than that of pure Degussa P25.
- The photo-activity of iron-doped TiO2 under visible light irradiation was evaluated.
- The pseudo-first-order rate kinetics indicates a rate-limiting step involving a chemical reaction.