Efficient Epoxidation of Alkenes using New Organometallic Catalysts ((E)-2,6-dimethoxy-4-((2-(5-methyl-1,3,4-thiadiazol-2-yl)hydrazono)methyl)phenol M: Cr, Fe, Co, Cu): An Antimicrobial and Theoretical Study of Catalyst
Authors
Abstract
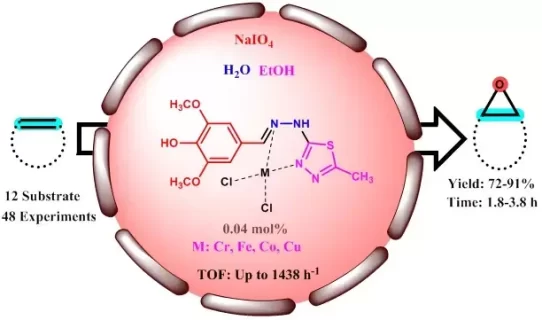
In this research, a novel approach for the epoxidation of alkene derivatives was constructed. Based on this fact, the appropriate ligand was provided from the reaction of hydrazine hydrate and 5-methyl-1,3,4-thiadiazole-2-thiol. Then, after preparation of the intermediate compound, the treatment of the pointed compound and 4-hydroxy-3,5-dimethoxybenzaldehyde in the presence of a few drops of glacial acetic acid for 3h was done in order to a final ligand. Then, the synthesized ligand was metallated using Cr, Fe, Co, and Cu to obtain a series of organometallic catalysts. The synthesized organometallic catalysts were analyzed using CHNS analysis, FT-IR spectroscopy, and magnetic susceptibility. Then, the organometallic complexes containing (Chromium, Iron, Cobalt, and Copper) are applied in the epoxidation of various alkenes to provide corresponding target products and provide moderate to good yields. The prepared organometallic complexes were investigated relative to the reusability and loss of metal into the medium of the reaction. In addition, the optimized chemical structures of organometallic complexes were investigated using DFT calculation.