An Iron-enhanced nanocone assisted drug delivery of Aspirin: DFT assessments
Authors
-
Ali Ghasemi Gol
 1
1
-
Jafar Akbari
*
 1
1
-
Mehdi Khalaj
 1
1
Abstract
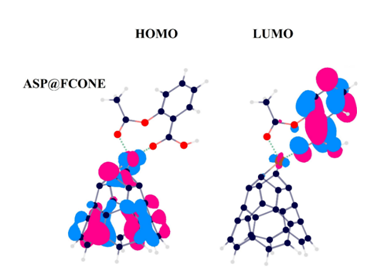
By the importance of customizing appropriate carriers for the specific drugs to approach a successful drug delivery process, the drug delivery of aspirin (ASP) was assessed by the assistance of an iron-enhanced nanocone (FCONE), using density functional theory (DFT) calculations. ASP, CONE, and FCONE models were optimized to be prepared for involving in bimolecular interactions to form ASP@CONE and ASP@FCONE complexes along with re-optimization calculations and vibrational frequency confirmations. Benefits of the enhanced FCONE model were seen for better interacting with the ASP counterpart comparing with the CONE and ASP interactions within the evaluated values of -26.35 and -10.07 kcal/mol for the corrected binding energies to yield a meaningful “recovery time” term. Additionally, the electronic molecular orbital features showed a priority for a better detection of ASP counterpart by the FCONE, in which the variations of energy gap values yielded a meaningful “conductance rate” especially for the ASP@FCONE complex. As a consequence, the recognized models of ASP@CONE and ASP@FCONE complexes were learned by a better advantage of enhanced FCONE model to be worked a s better proposed carrier for the ASP drug delivery process.
Graphical Abstract
Keywords
References
- Sultana A., Zare M., Thomas V., Kumar T.S., Ramakrishna S., (2022), Nano-based drug delivery systems: Conventional drug delivery routes, recent developments and future prospects. Med. Drug Discover. 15: 100134. https://doi.org/10.1016/j.medidd.2022.100134
- Rabiee F., Mehralizadeh N., Jalalinezhad S., Ebrahimi Z., Jamali S., (2022), Evaluation of the effects of nanoparticles in the treatment of diabetes mellitus: A systematic review and meta-analysis. Int. J. Sci. Res. Dent. Med. Sci. 4: 191-195.
- Shabani M., Ghiasi R., Zare K., Fazaeli R., (2021), The interaction between carboplatin anticancer drug and B12N12 nano-cluster: a computational investigation. Main Group Chem. 20: 345-354. https://doi.org/10.3233/MGC-210051
- Chavda V. P., Patel A. B., Mistry K. J., Suthar S. F., Wu Z. X., Chen Z. S., Hou K., (2022), Nano-drug delivery systems entrapping natural bioactive compounds for cancer: Recent progress and future challenges. Front. Oncol. 12: 867655-867658. https://doi.org/10.3389/fonc.2022.867655
- Aghazadeh H., Tamaddon F., Ouni M., Taheri P., Sangchooli T., (2023), Microencapsulation of herbal bioactive drug by Chlorella Vulgaris microalgae as a nano-formulation for drug delivery to cells. Eurasian Chem. Commun. 5: 327-334.
- Ghiasi R, Sofiyani M. V., Emami R., (2021), Computational investigation of interaction of titanocene dichloride anti-cancer drug with carbon nanotube in the presence of external electric field. Biointerface Res. Appl. Chem. 11: 12454-12461. https://doi.org/10.33263/BRIAC114.1245412461
- Fazlzadeh A., Sabbagh Seddigh S., Sabbagh Seddigh Sh., (2023), Evaluation of the diagnostic accuracy of carbon nanoparticle suspensions in sentinel lymph node biopsy of breast cancer: A systematic review and meta-analysis. Int. J. Sci. Res. Dent. Med. Sci. 5: 154-163.
- Qasim M. A., Yaaqoob L. A., (2023), Evaluation of antibacterial activity of iron oxide nanoparticles synthesis by extracellular lactobacillus against pseudomonas aeruginosa. J. Med. Chem. Sci. 6: 1100-1111.
- Mukherjee P., Mandal K., Kumar A., (2021), The titbits of multi-drug resistant organisms reigning in the diabetic foot ulcers: regional epidemiology from a tertiary care hospital of eastern India. Int. J. Sci. Res. Dent. Med. Sci. 3: 6-11.
- Bhuyan A., Ahmaruzzaman M., (2023), Recent advances in new generation nanocomposite materials for adsorption of pharmaceuticals from aqueous environment. Environ. Sci. Pollut. Res. 30: 39377-39417. https://doi.org/10.1007/s11356-023-25707-0
- Moayeripour S. S., Behzadi R., (2023), Experimental investigation of the effect of titanium nano-particles on the properties of hydrophobic self-cleaning film. Eurasian Chem. Commun. 5: 303-316.
- Farhadi B., Ebrahimi M., Morsali A., (2022), Pre-concentration and sensitive determination of propranolol and metoprolol using dispersive solid-phase microextraction and high-performance liquid chromatography in biological, wastewater, and pharmaceutical samples. Chem. Methodol. 6: 750-761.
- Liu J., Liu Z., Pang Y., Zhou H., (2022), The interaction between nanoparticles and immune system: application in the treatment of inflammatory diseases. J. Nanobiotechnol. 20: 127-130. https://doi.org/10.1186/s12951-022-01343-7
- Kazemi Z., Ghiasi R., Jamehbozorgi S., (2018), Analysis of the interaction between the C20 cage and cis-Ptcl2(NH3)2: A DFT investigation of the solvent effect, structures, properties, and topologies. J. Struct. Chem. 59: 1044-1051. https://doi.org/10.1134/S0022476618050050
- Alizadeh S., Nazari Z., (2022), Amphetamine, methamphetamine, morphine@AuNPs kit based on PARAFAC. Adv. J. Chem. A. 5: 253-262.
- Esfahani S., Akbari J., Soleimani-Amiri S., Mirzaei M., Ghasemi Gol A., (2023), Assessing the drug delivery of ibuprofen by the assistance of metal-doped graphenes: insights from density functional theory. Diam. Relat. Mater. 135: 109893-109896. https://doi.org/10.1016/j.diamond.2023.109893
- Ghiasi R., Valizadeh A., (2023), Computational investigation of interaction of a cycloplatinated thiosemicarbazone as antitumor and antiparasitic agents with B12N12 nano-cage. Results Chem. 5: 100768. https://doi.org/10.1016/j.rechem.2023.100768
- Ali F., Fazal S., Iqbal N., Zia A., Ahmad F., (2023), PANI-based nanocomposites for the removal of dye from wastewater. Asian J. Nanosci. Mater. 6: 106-124.
- Waheed S., Li Z., Zhang F., Chiarini A., Armato U., Wu J., (2022), Engineering nano-drug biointerface to overcome biological barriers toward precision drug delivery. J. Nanobiotechnol. 20: 395-398. https://doi.org/10.1186/s12951-022-01605-4
- Salehi Kahrizsangi F., Mehrafar N., Pezhman Ghadami F., Rabiee F., Shariati Y., (2022), Evaluation of the clinical outcome of nab-paclitaxel on multiple primary malignancies: a systematic review and meta-analysis. Int. J. Sci. Res. Dent. Med. Sci. 4: 183-190.
- Heidaripour A., Salmani F., Barati T., (2023), Synthesis of coral-Like ZnO nanostructures with high and wide absorption range. Asian J. Green Chem. 7: 140-148. https://doi.org/10.2139/ssrn.4413255
- Chala G., (2023), Review on green synthesis of iron-based nanoparticles for environmental applications. J. Chem. Rev. 5: 1-14.
- Ghadami P., Mehrafar N., Rajabi H., Rabiee F., Eghbalifard N., (2023), Evaluation of the effect of mesenchymal stem cells on breast cancer migration and metastasis: A systematic review and meta-analysis. Int. J. Sci. Res. Dent. Med. Sci. 5: 164-170.
- Yang N., Zhang G., Li B., (2008), Carbon nanocone: a promising thermal rectifier. Appl. Phys. Lett. 93: 243111. https://doi.org/10.1063/1.3049603
- Kadhim M. M., Sheibanian N., Ashoori D., Sadri M., Tavakoli-Far B., Khadivi R., Akhavan-Sigari R., (2022), The drug delivery of hydrea anticancer by a nanocone-oxide: Computational assessments. Comput. Theor. Chem. 1215: 113843. https://doi.org/10.1016/j.comptc.2022.113843
- Kumar A., Sayyed M. I., Sabugaa M. M., Al-Bahrani M., Sharma S., Saadh M. J., (2023), A DFT study on effective detection of ClCN gas by functionalized, decorated, and doped nanocone strategies. RSC Adv. 13: 12554. https://doi.org/10.1039/D3RA01231J
- Moghaddam F. A., Babazadeh M., Vessally E., Ghasemi E., Shendy S. A., (2023), An efficient HCN gas sensor by functionalized, decorated, and doped nanocone strategy: Theoretical study. Inorg. Chem. Commun. 156: 111118. https://doi.org/10.1016/j.inoche.2023.111118
- Söğütlü İ., Arshadi S., Mahmood E. A., Abbasi V., Kamalinahad S., Vessally E., (2023), In silico investigation of metalophthalocyanine substituted in carbon nanocones (TM-PhCCNC, TM= Sc2+, Cr2+, Fe2+ and Zn2+) as a promising sensor for detecting N2O gas involved in Covid-19. J. Mol. Struct. 1284: 135263. https://doi.org/10.1016/j.molstruc.2023.135263
- Ashwini T., Narayan R., Shenoy P. A., Nayak U. Y., (2022), Computational modeling for the design and development of nano based drug delivery systems. J. Mol. Liq. 368: 120596. https://doi.org/10.1016/j.molliq.2022.120596
- Mirzaei M., Hadipour N., Gülseren O., (2020), Cubane cluster surface for pyrimidine nucleobases relaxation: DFT approach. Int. J. Nano Dimens. 12: 135-144.
- Fekri M. H., Bazvand R., Soleymani M., Razavi Mehr M., (2020), Adsorption of metronidazole drug on the surface of nano fullerene C60 doped with Si, B and Al: A DFT study. Int. J. Nano Dimens. 11: 346-354.
- Kazemi Z., Ghiasi R., Jamehbozorgi S., (2020), The interaction of 5-fluorouracil with graphene in presence of external electric field: a theoretical investigation. Adsorption. 26: 905-911. https://doi.org/10.1007/s10450-019-00140-3
- Green G. A., (2001), Understanding NSAIDs: From aspirin to COX-2. Clin. Corner. 3: 50-59. https://doi.org/10.1016/S1098-3597(01)90069-9
- Orkaby A. R., Yang L., Dufour A. B., Travison T. G., Sesso H. D., Driver J. A., Djousse L., Gaziano J. M., (2021), Association between long-term aspirin use and frailty in men: The physicians' health study. J. Gerontol. A. 76: 1077-1083. https://doi.org/10.1093/gerona/glaa233
- Angiolillo D. J., Prats J., Deliargyris E. N., Schneider D. J., Scheiman J., Kimmelstiel C., Steg P. G., Alberts M., Rosengart T., Mehran R., Bhatt D. L., (2022), Pharmacokinetic and pharmacodynamic profile of a novel phospholipid aspirin formulation. Clin. Pharmacokin. 61: 465-479. https://doi.org/10.1007/s40262-021-01090-2
- Mubder N. S., Al-Tameemi M., Mahmood H., Salman N. K., Al-Neshmi H., (2022), Micro spectrophotometric determination and cloud point extraction of aspirin with iron (III) in pure form and pharmaceutical drugs. Chem. Methodol. 6: 569-570.
- Khudhair F. A., Ali S. H., Al-Zubeidi K. A., (2022), Impact of RAGE gene rs80096349(C>T), rs1035798(C>T), and rs184003(G>T) polymorphisms on non-response to aspirin in Iraqi patients with coronary artery disease. J. Med. Chem. Sci. 6: 1582-1597.
- Kanwal F., Ma M., Rehman M. F., Khan F. U., Elizur S. E., Batool A. I., Wang C. C., Tabassum T., Lu C., Wang Y., (2022), Aspirin repurposing in folate-decorated nanoparticles: Another way to target breast cancer. Front. Mol. Biosci. 8: 788279. https://doi.org/10.3389/fmolb.2021.788279
- Li S., Yang Y., Xiao W., Yin L., Liu L., Liu Y., Sun Y., Chen Z., (2023), Preparation and characterization of nano-hydroxyapatite/aspirin/polyvinyl alcohol/gelatin/sodium alginate hydrogel scaffolds. Chinese J. Tissue Eng. Res. 27: 3956-3959.
- Wang K., Shen R., Meng T., Hu F., Yuan H., (2022), Nano-drug delivery systems based on different targeting mechanisms in the targeted therapy of colorectal cancer. Molecules. 27: 2981-2985. https://doi.org/10.3390/molecules27092981
- Shokuhi Rad A., (2023), A new strategy for making a sensitive sensor for aspirin drug: first-principles investigations by using pure and metal-doped BN nano-heterostructures. J. Biomol. Struct. Dyn. 2023: 1-13. https://doi.org/10.1080/07391102.2023.2194995
- Ghasemi Gol A., Akbari J., Khalaj M., Mousavi-Safavi S. M., Esfahani S., Farahani N., (2023), DFT investigation of a Zn-doped carbon nanocone for the drug delivery of methylated aspirins. Comput. Theor. Chem. 1220: 113976. https://doi.org/10.1016/j.comptc.2022.113976
- Gaussian 09, Revision D.01, Frisch M. J., Trucks G. W., Schlegel H. B., Scuseria G. E., Robb M. A., Cheeseman J. R., Scalmani G., Barone V., Petersson G. A., Nakatsuji H., Li X., Caricato M., Marenich A., Bloino J., Janesko B. G., Gomperts R., Mennucci B., Hratchian H. P., Ortiz J. V., Izmaylov A. F., Sonnenberg J. L., Williams-Young D., Ding F., Lipparini F., Egidi F., Goings J., Peng B., Petrone A., Henderson T., Ranasinghe D., Zakrzewski V. G., Gao J., Rega N., Zheng G., Liang W., Hada M., Ehara M., Toyota K., Fukuda R., Hasegawa J., Ishida M., Nakajima T., Honda Y., Kitao O., Nakai H., Vreven T., Throssell K., Montgomery J. A., Jr., Peralta J. E., Ogliaro F., Bearpark M., Heyd J. J., Brothers E., Kudin K. N., Staroverov V. N., Keith T., Kobayashi R., Normand J., Raghavachari K., Rendell A., Burant J. C., Iyengar S. S., Tomasi J., Cossi M., Millam J. M., Klene M., Adamo C., Cammi R., Ochterski J. W., Martin R. L., Morokuma K., Farkas O., Foresman J. B., Fox D. J., (2016), Gaussian, Inc., Wallingford CT.
- Chai J. D., Head-Gordon M., (2008), Long-range corrected hybrid density functionals with damped atom-atom dispersion corrections. Phys. Chem. Chem. Phys. 10: 6615-6620. https://doi.org/10.1039/b810189b
- Rassolov V. A., Ratner M. A., Pople J. A., Redfern P. C., Curtiss L. A., (2001), 6‐31G* basis set for third row atoms. J. Comput. Chem. 22: 976-984. https://doi.org/10.1002/jcc.1058
- Bader R. F., Nguyen-Dang T. T., (1981), Quantum theory of atoms in molecules-Dalton revisited. Adv. Quantum Chem. 14: 63-124. https://doi.org/10.1016/S0065-3276(08)60326-3
- Lu T., Chen F., (2012), Multiwfn: A multifunctional wavefunction analyzer. J. Comput. Chem. 33: 580-592. https://doi.org/10.1002/jcc.22885
- Chemcraft - graphical software for visualization of quantum chemistry computations. Version 1.8, build 654. https://www.chemcraftprog.com.
- O'boyle N. M., Tenderholt A. L., Langner K. M., Cclib: A library for package independent computational chemistry algorithms. J. Comput. Chem. 29: 839-845. https://doi.org/10.1002/jcc.20823
- Adekoya O. C., Adekoya G. J., Sadiku E. R., Hamam Y., Ray S. S., (2022), Application of DFT calculations in designing polymer-based drug delivery systems: An overview. Pharmaceutics. 14: 1972-1975. https://doi.org/10.3390/pharmaceutics14091972
- Dey D., De D., (2018), First principle study of structural and electronic transport properties for electrically doped zigzag single wall GaAs nanotubes. Int. J. Nano Dimens. 9: 134-144.
- Cosme-Pecho R. D., Hajali N., Tapia-Silguera R. D., Yassen L., Alwan M., Jawad M. J., Castro-Cayllahua F., Mirzaei M., Akhavan-Sigari R., (2023), Molecular insights into the sensing function of an oxidized graphene flake for the adsorption of avigan antiviral drug. Comput. Theor. Chem. 1227: 114240. https://doi.org/10.1016/j.comptc.2023.114240
- Sadjadi M. S., Sadeghi B., Zare K., (2007), Natural bond orbital (NBO) population analysis of cyclic thionylphosphazenes, [NSOX (NPCl2)2]; X=F(1), X=Cl(2). J. Mol. Struct. THEOCHEM. 817: 27-33. https://doi.org/10.1016/j.theochem.2007.04.015
- Davidson E. R., Chakravorty S. J., (1994), A possible definition of basis set superposition error. Chem. Phys. Lett. 217: 48-54. https://doi.org/10.1016/0009-2614(93)E1356-L
- Heidari M., Solimannejad M., (2022), Sensing behavior of porous B6N6 boron nitride covalent organic framework toward cathinone drugs: A DFT study. Inorg. Chem. Commun. 146: 110205. https://doi.org/10.1016/j.inoche.2022.110205