In Vitro Evaluation, Molecular Docking, and Lipinski’s Rule Analysis of a new Triazinone-Based Schiff Base for Potential Pharmacological Applications
- Department of Chemistry, Ya.C. Islamic Azad University, Yazd, Iran.
- Fachbereich Chemie der Universität Marburg, Marburg, Germany.

Received: 2023-08-07
Revised: 2023-09-25
Accepted: 2023-09-28
Published in Issue 2023-09-30
Copyright (c) 2025 Copyright © 2024, The Author(s), under exclusive licence to Islamic Azad University

This work is licensed under a Creative Commons Attribution 4.0 International License.
How to Cite
Abstract
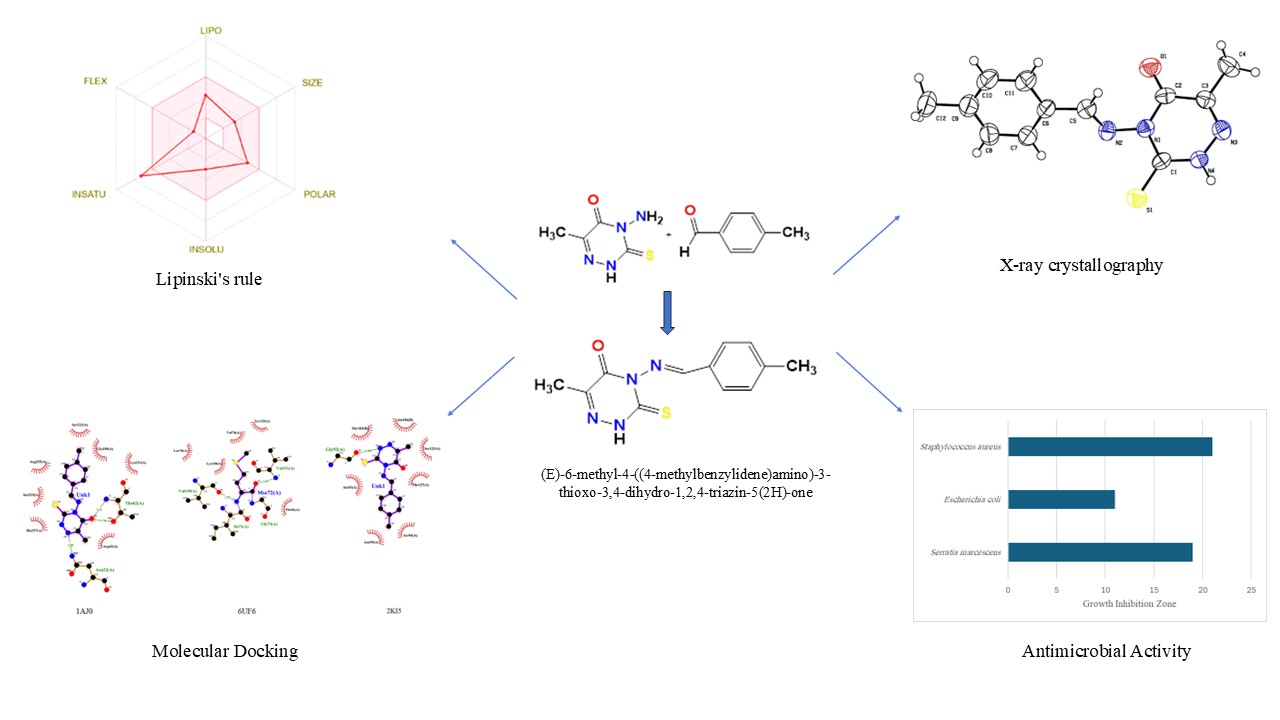
(E)-6-methyl-4-((4-methylbenzylidene)amino)-3-thioxo-3,4-dihydro-1,2,4-triazin-5(2H)-one (1), was synthesized from the reaction of 4-amino-3-mercapto-6-methyl-5-oxo-1,2,4-triazine (AMTTO) and 4-methyl benzaldehyde under microwave conditions and characterized through ¹H-NMR, FTIR spectroscopy, and single-crystal X-ray diffraction. The Hirshfeld surface was analyzed and results indicate a significant effect of hydrogen interactions and their role in the stability of the crystal lattice of the 1. The in vitro antibacterial activities were evaluated using the disk diffusion technique. 1 displays moderate to significant effect on bacterial strains. In silico Molecular docking simulations were performed to investigate the interaction 1 with the active site of three target proteins. Discovery Studio Visualizer and LigPlot+ software was used to illustrate molecular docking results. The best confirmation from molecular docking studies shows that the free energies of binding for the studied ligands were -6.7, -6.6, and -6.6 kcal/mol, as were the creation of hydrogen bonds between the studied compound and the target proteins. In silico prediction of physicochemical properties (Lipinski's rule of five) and bioavailability radar were determined by the SwissADME database. The physicochemical property analysis revealed that 1 has a bioavailability score of 0.55. The results of this study indicate that 1 could be a lead compound for developing antimicrobial and antiviral compounds.
Keywords
- Thiotriazine Schiff-base compound,
- Antibacterial Activities,
- Molecular Docking,
- Lipinski's Rules
References
- Janowska, S., Khylyuk, D., Andrzejczuk, S., Wujec, M.: Design, Synthesis, Antibacterial Evaluations and In Silico Studies of Novel Thiosemicarbazides and 1,3,4-Thiadiazoles, Molecules (2022). https://doi.org/10.3390/molecules27103161
- Kanso F., Khalil A., Noureddine H., El-Makhour Y., Therapeutic perspective of thiosemicarbazones derivatives in inflammatory pathologies: A summary of in vitro/in vivo studies, International Immunopharmacology, (2021) https://doi.org/10.1016/j.intimp.2021.107778
- Greenbaum, D. C.; Mackey, Z.; Hansell, E.; Doyle, P.; Gut, J.; Caffrey, C. R.; Lehrman, J.; Rosenthal, P. J.; McKerrow, J. H.; Chibale, K. Synthesis and Structure−Activity Relationships of Parasiticidal Thiosemicarbazone Cysteine Protease Inhibitors against Plasmodium falciparum, Trypanosoma brucei, and Trypanosoma cruzi. J. Med. Chem. 2004, 47, 6669–6678. https://doi.org/10.1021/jm030549j
- Ghassemzadeh, M., Tabatabaee,M., Mirrokni, Z., Neumüller, B.: Synthesis, Characterization and Crystal Structure of Dimeric {[(AMTTO)2Ag]NO3}2 (AMTTO 4-Amino-6-methyl-1,2,4-triazine-3(2H)-thione-5-one), Z. Anorg. Allg. Chem. (2005) DOI: 10.1002/zaac.200500012.
- Bazyari, P., Tabatabaee, M., Nasirizadeh, N., Dušek, M., Eigner, V.: A Unique Example of a Co crystal of [Co(AMTTO)2(H2O)2](NO3)2 and [Co(AMTTO)2(CH3CN)2](NO3)2 AMTTO=4 amino 5 methyl 1,2,4 triazol 3(2H) thione), J. Chem. Crystal. (2021) https://doi.org/10.1007/s10870-021-00882-5
- Ghassemzadeh, M., Bahemmat, S., Tabatabaee, M., Nassiri, S., Neumüller, B.: Synthesis, characterization and crystal structures of palladium(II) complexes containing neutral, one and twofold deprotonated 1,2,4-triazine species, Polyhedron (2011) doi:10.1016/j.poly.2011.03.048.
- Adhamia, F., Tabatabaee, M., Gassemzadehc, M., Neumüllerd,B.: A New Complex as a Dimer of a Silver ion with AMTTO and Phosphane Ligands. The Crystal Structure of {[(AMTTO)Ag(PPh3)2]NO3}2 · 0.5CH3OH· 0.5H2O, Z. Anorg. Allg. Chem. (2008) DOI: 10.1002/zaac.200800072.
- Tabatabaee, M. Ghassemzadeh, M., Neumüller, B., Syntheses, Characterization and X-ray Structure of the First Silver(I) Complexes with 4-Amino-3-thioxo-3,4-dihydro-1,2,4-triazin-5(2H)-one, Z. Anorg. Allg. Chem. (2009) DOI: 10.1002/zaac.200801417.
- Ghassemzadeh, M., Pooramini, M.M., Tabatabaee, M., Heravi, M.M., Neumüller, B.: Syntheses, Characterization and X-Ray Structures of the first Copper(I) and Silver(I) Complexes with ATT (ATT 6-Aza-2-thiothymine). Z. Anorg. Allg. Chem. (2004) DOI: 10.1002/zaac.200300362.
- Ghassemzadeh, M., Mirza-Aghayan, M., Neumüller, B.: Syntheses and characterization of the first platinum complex and new palladacycles of N,S-chelating agent in ‘‘triplex’’ form: molecular structures of [(AMTTO)PtCl2]3 Æ 4.5THF and [(AMTTO)PdX2]3 Æ 8MeOH (X = Cl and Br) (AMTTO=4-amino-6-methyl-1,2,4-triazine-3-thione-5-one), Inorg. Chim. Acta, (2005) https://doi.org/10.1016/j.ica.2004.12.025.
- Adhami, F., Ghassemzadeh, M., Heravi, M.M., Taeb, A., Neumüller, B.: Synthesis and Crystal Structure of a New Silver(I) Complex, Z. Anorg. Allg. Chem. (1999) https://doi.org/10.1002/(SICI)1521-3749(199909)625:9<1411
- Ghassemzadeh, M., Firouzi, R., Shirkhani, S., Amiri,S.R., Neumüller, B.:New dinuclear copper(I) metallacycles containing bis-Schiff base ligands fused with two 1,2,4-triazole rings: Synthesis, characterization, molecular structures and theoretical calculations. Polyhedron (2014) https://doi.org/10.1016/j.poly.2013.11.030
- Ghassemzadeh, M., Bahemmat, S., Mahmoodabadi, M., Rezaii-Rad, B., Monfared, H.H., Mottefakeri, E., Neumüller, B.: New mono- and binuclear Pd(II) complexes containing 1,2,4-triazole moieties. Polyhedron (2010) https://doi.org/10.1016/j.poly.2010.08.012.
- Tsacheva, I., Todorova, Z., Momekova, D., Momekov, G., Koseva, N.: Pharmacological Activities of Schiff Bases and Their Derivatives with Low and High Molecular Phosphonates. Pharmaceuticals (2023) https://doi.org/10.3390/ph16070938
- Sinha, D., Tiwari, A.K., Singh, S., Shukla, G., Mishra, P., Chandra, H., Mishra, A.K.: Synthesis, characterization and biological activity of Schiff base analogues of indole-3-carboxaldehyde, Eur. J. Med. Chem. (2008) https://doi.org/10.1016/j.ejmech.2007.03.022
- Ceramella, J., Escopeta, D., Catalano, A., Cirillo, F., Lappano, R., Sinicropi, M.S.: A Review on the Antimicrobial Activity of Schiff Bases: Data Collection and Recent Studies. Antibiotics (2022) https://doi.org/10.3390/antibiotics11020191
- Munawar, K.S., Haroon, S.M., Hussain, S.A., Raza, H.: Schiff bases: Multipurpose pharmacophores with extensive biological applications. J. Basic Appl. Sci. 14, 217–229 (2018)
- Iacopetta, D., Lappano, R., Mariconda, A., Ceramella, J., Sinicropi, S.M., Saturnino, C., Talia, M., Cirillo, F., Martinelli, F., Puoci, F., Rosano, C., Longo, P., Maggiolini, M.: Newly synthesized imino-derivatives analogues of resveratrol exert inhibitory effects in breast tumor cells. Int. J. Mol. Sci. (2020) https://doi.org/10.3390/ijms21207797.
- Kumar, K.S., Ganguly, S., Veerasamy, R., De Clercq, E.: Synthesis, antiviral activity and cytotoxicity evaluation of Schiff bases of some 2-phenyl quinazoline-4 (3) H-ones. Eur. J. Med. Chem. (2010) DOI: 10.1016/j.ejmech.2010.07.058.
- Kargar, H., Fallah-Mehrjardi, M., Ashfaq, M., Munawar, K., Tahir, M.N., Behjatmanesh-Ardakani, R., Rudbari, H.A., Adabi Ardakani, A., Sedighi-Khavidak, S.: Zn(II) complexes containing O,N,N,O-donor Schiff base ligands: synthesis, crystal structures, spectral investigations, biological activities, theoretical calculations and substitution effect on structures, J. Coord. Chem. (2021) https://doi.org/10.1080/00958972.2021.1990271.
- Silver, L.L.: Challenges of Antibacterial Discovery. Clin Microbiol Rev. (2011). https://doi.org/10.1128/cmr.00030-10
- Tabatabaee, M., Ghassemzadeh, M., Zarabi, B., Neumüller, B.: Z. Naturforsch. Synthesis and Crystal Structure of Schiff Bases Based on AMTTO (AMTTO = 4-Amino-6-methyl-3-thio-3,4-dihydro-1,2,4-triazin5(2H)-one), Z. Naturforsch. (2006) https://doi.org/10.1515/znb-2006-1116.
- 21. Tabatabaee, M., Ghassemzadeh, M., Zarabi, B., Heravi, M.M., Anary-Abbasinejad, M., Neumüller, B.: Solvent-Free Microwave Synthesis of (Aryland Heteroaryl-methylene)-amino Derivatives of 4-Amino-6-methyl-5-oxo-3-thioxo-2H-1,2,4-triazine and 4-Amino-5-methyl-3-thioxo-2H-1,2,4-triazole: Crystal Structure of 6-Methyl-4-(3-nitrobenzylideneamino)- 5-oxo-3-thioxo-2H-1,2,4-triazine Phosphorus Sulfur Silicon Relat. Elem. (2007) DOI: 10.1080/10426500601047461
- Shirinkam, B.,Tabatabaee, M., Kukovec, B.-M., Oliver, C.L., Ghassemzadeh, M.: Preparation, spectroscopic characterization, and crystal structure of a mixed-ligand silver(I) complex with 1,2,4-triazole-based Schiff base and triphenylphosphine, Monatsh. Chem. 92014) DOI 10.1007/s00706-014-1256-z
- Ghassemzadeh, M., Tabatabaee, M., Pooramini, M. M., Heravi, M.M., Eslami, A., Neumüller, B.: Synthesis, Characterization of a Novel 1,2,4-Thiotriazine Derivative (CAMTTO), and Copper(I) and Silver(I) Complexes thereof (CAMTTO {(4-[(4-Chlorobenzylidene)-amino]-6-methyl-3-thioxo-[1,2,4]- triazin-3,4-dihydro(2H)-5-one)} Z. Anorg. Allg. Chem. (2006) DOI: 10.1002/zaac.200500334
- Bazyari, P., Tabatabaee, M., Nasirizadeh, N., Dušek, M., Eigner, V.: Crystal and Molecular Structures of Mononuclear Coordinated Copper(I) Complexes with Thio-triazole and Thio-triazine based Schiff base Ligands Z. Anorg. Allg. Chem. (2020) DOI: 10.1002/zaac.202000191
- Tabatabaee, M., Ghassemzadeh, M., Sadeghi, A., Shahriary, M., Neumüller, B.: A. Rothenberger, Synthesis, Characterization and X-ray Structures of AMTT-Type Schiff bases and two CuI Complexes (AMTT 4-amino-5-methyl-2H-1,2,4-triazole-3(4H)-thione) Z. Anorg. Allg. Chem. (2009) DOI: 10.1002/zaac.200800366.
- Ghaneian, M. T., Tabatabaee, M., Ehrampoush, M.H., Jebali, A., Hekmatimoghaddam, S.H., Fallahzadeh, H., Fallahzadeh, R.A.: Synthesis of Ag(I) and Cu(I) Complexes With 4-Amino-5-Methyl-2h-1,2,4-Triazole-3(4h)-Thione Ligand as Thiocarbohydrazide Derivatives and Their Antimicrobial Activity, Pharm. Chem. J. (2015) 10.1007/s11094-015-1258-0
- Tahriri, M., Yousefi, M., Mehrani, K., Tabatabaee, M., Dehghani Ashkezari, M.: Synthesis, Characterization and Antimicrobial Activity of Two Novel Sulfonamide Schiff, Base Compounds, Pharm. Chem. J. (2017) DOI 10.1007/s11094-017-1626-z.
- Dornow, A., Menzel, H., Marx, P.: Chem. Ber. 97, 2173-2175 (1964).
- Sheldrick, G. M.: SHELXL-97, Program for Crystal Structure Solution and Refinement Göttingen, (1997)
- Sheldrick, G.M.:SHELXL-2018 Program for Crystal Structure Refinement. University of Göttingen, (2018)
- Spackman, P.R., Turner, M. J., McKinnon, J.J., Wolff, S.K., Grimwood, D.J., Jayatilaka, D., Spackman, M.A.: Crystal Explorer: a program for Hirshfeld surface analysis, visualization and quantitative analysis of molecular crystals. J. Appl. Cryst. (2021) https://doi.org/10.1107/S1600576721002910
- Protein Data Bank. Available from: https://www.rcsb.org.
- Computed Atlas of Surface Topography of proteins (CASTp). Available from: http://sts.bioe.uic.edu/castp/index.html?1aj0.
- http://www.scfbio-iitd.res.in/
- Zhang, A., Li, W., Liu, X., Wu, M., Xuan, G.: Biological Evaluation and in Silico Studies of Several Substituted Benzene Sulfonamides as Potential Antibacterial Agents. Journal of Physics (2022) https://doi.org/10.1088/1742-6596/1624/2/022058
- Varma, M.V., Perumal, O.P., Panchagnula, R.: Functional role of P-glycoprotein in limiting peroral drug absorption: optimizing drug delivery. Curr. Opin. Chem. Biol. (2006) https://doi.org/10.1016/j.cbpa.2006.06.015
- Roskoski, R.: Properties of FDA-approved small molecule protein kinase inhibitors. Pharmacological Research. (2019) https://doi.org/10.1016/j.phrs.2019.03.006
- Lipinski, C. A., Lombardo, F., Dominy, B. W., Feeney, P. J.: Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Deliv. Rev., (2001) https://doi.org/10.1016/S0169-409X(00)00129-0
- Hughes, J. D., Blagg, J., Price, D. A., Bailey, S., Decrescenzo, G. A., Devraj, R. V., Weidolf, L.: Physiochemical drug properties associated with in vivo toxicological outcomes. Bioorg. Med. Chem. Lett (2008) https://doi.org/10.1016/j.bmcl.2008.07.086

 10.57647/pibm.2023.122316
10.57647/pibm.2023.122316