Synthesis of SO4/ZrO2 Catalyst and its Application in the Conversion of Ethanol to Diethyl Ether
Authors
Abstract
SO4/ZrO2 heterogeneous acid catalyst was prepared by wet impregnation method from ZrO2 precursor involved variations in H2SO4 concentration (0.5; 1.0; 1.5 M) and calcination temperature (400, 500, 600 ℃) to yield catalyst with the highest acidity value. The catalysts produced were characterized using Fourier Transform Infrared (FTIR) spectrometer, X-Ray Diffractometer (XRD), Scanning Electron Microscope-Energy Dispersive X-Ray (SEM-EDX), Thermogravimetry and Differential Scanning Calorimeter (TGA-DSC), Gas Sorption Analyzer (GSA), and acidity test using the gravimetric method with ammonia vapor. The catalyst used to observe activity and selectivity in the dehydration reaction of ethanol to diethyl ether (DEE) was SO4/ZrO2 catalyst with the highest total acidity. The liquid product from the dehydration of ethanol was analyzed using Gas Chromatography (GC). The ZS‐1.5‐500 catalyst showed the best activity and selectivity in the dehydration reaction of ethanol to DEE at a temperature of 225 ℃, yielding 49.85% (w/w) ethanol conversion and a 1.62% DEE selectivity.
Highlights
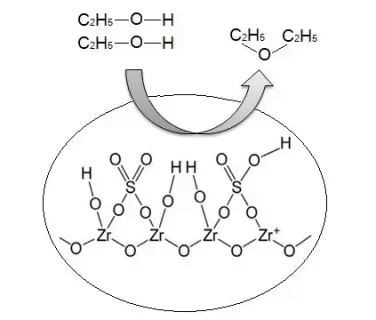
- Sulfation of zirconia results in higher strength of Brønsted and Lewis acid sites on its surface.
- Modified zirconia using sulfate increased the catalytic activity and selectivity of catalyst towards diethyl ether (DEE).
- The highest DEE content obtained was 1.62% using a ZS-1.5-500 catalyst which has the highest acidity at a reaction temperature of 225 ℃.