Performance of Ni, Pt, and Pd Monometal and Ni-Pt Bimetal onto Activated Carbon for Hydrocracking of Castor Oil
Authors
Abstract
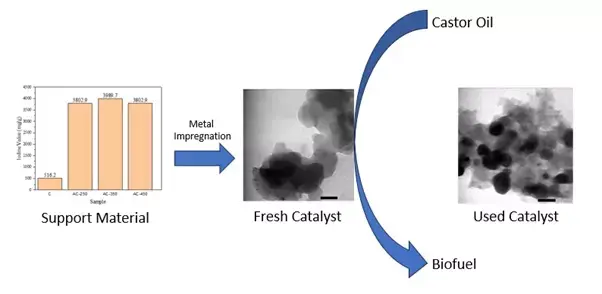
The development of high-performance hydrotreating catalysts has been a challenging pursuit within the catalyst research field. In this study, activated carbon was synthesized chemically, utilizing oxygen gas as the activator and Merbau wood as the precursor. Subsequently, the activated carbon was impregnated with both mono (Ni, Pt, Pd) and bimetallic (NiPt) species. Physical activation employing oxygen gas was employed in the preparation of the activated carbon. Notably, the optimum activation temperature using oxygen gas was identified at 350°C, aligning with the peak iodine value of 3989.7 mg/g. Subsequently, the activated carbon served as a highly efficient support material for the hydrocracking of castor oil. Among the investigated catalysts, the NiPt/AC catalyst emerged as the most promising, achieving a remarkable liquid fraction conversion of 88.73 wt%. However, it is crucial to acknowledge that the NiPt/AC catalyst exhibited limitations in terms of stability, experiencing sintering and performance degradation after only three usage cycles.
Highlights
- Little to no research journal use oxygen gas as activating agent in the making of activated carbon.
- The utilization of monometallic Ni, Pt, and Pd species on the AC support material revealed distinct mechanisms, wherein Ni/AC exhibited notable hydrodeoxygenation, while the noble metals, Pt and Pd, predominantly engaged in the cleavage of carbon-carbon bonds.
- Subsequent to the activation process with oxygen gas, a substantial increase in the iodine value of the activated carbon was observed, signifying an enhanced porosity and surface area.
- The investigation into the reusability of the NiPt/AC catalyst highlighted its limited stability and susceptibility to sintering phenomena, posing challenges for sustained catalytic performance.