Parametric and kinetic study for synthesis of biofuel additive using low-cost ion-exchange resin as a catalyst
Authors
Abstract
Levulinic acid (LA) is a valuable renewable resource that can serve as a versatile biomassderived
building block for the synthesis of organic chemicals, providing a sustainable alternative
to depleting fossil fuel resources. Catalytic esterification of LA with alcohol yields levulinate
esters, which find numerous applications including perfumes and fuel additives. In this study, the
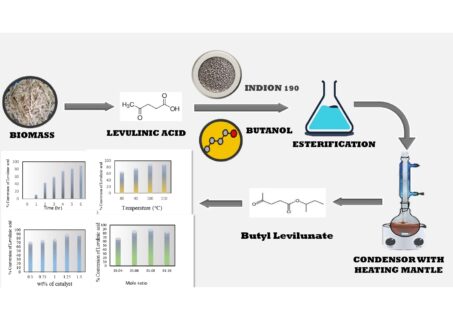
performance of Indion 190 as a solid acid catalyst was investigated for the esterification of LA
with n-butanol to produce n-butyl levulinate (BL). The effects of reaction time, catalyst loading,
temperature, and molar ratio of LA to butanol were examined One Factor at a Time (OFAT) to
optimize levulinic acid conversion. The highest conversion of LA, which was 92.59%, was attained
with a reaction time of 6 hours at 100 ◦C, utilizing a catalyst loading of 1.25 wt% Indion 190 for a
molar ratio (LA: butanol) of 1:8. In the kinetic study of the reaction, an activation energy of 65.5
kJ/mol was obtained. Indion 190 proved to be a promising, cost-effective solid acid catalyst for the
production of levulinate esters, providing an eco-friendly pathway for the continuous production
of butyl levulinate.
Highlights:
· In this study, the performance of Indion 190 as a solid acid catalyst was investigated for the esterification of LA with n-butanol to produce n-butyl levulinate (BL).
· The effects of reaction time, catalyst loading, temperature and molar ratio of LA to butanol were examined One Factor At a Time (OFAT) to optimize levulinic acid conversion.
· The highest conversion of LA, which was 92.59%, was attained with a reaction time of 6 hours AT 100 °C, utilizing a catalyst loading of 1.25 wt% Indion 190 for a molar ratio (LA:butanol) of 1:8.
· The kinetic study of the reaction was done using power law model and an activation energy of 65.5 kJ/mol was obtained.
· Indion 190 proved to be a promising, cost-effective solid acid catalyst for the production of levulinate esters, providing an eco-friendly pathway for the continuous production of butyl levulinate.