Optimization of main parameters affecting activity and octane number produced from catalytic isomerization of n-heptane using response surface methodology
Authors
Abstract
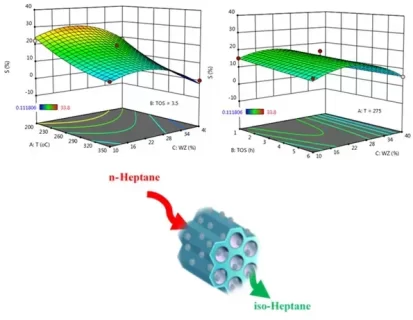
In this work, the Response Surface Methodology (RSM) was investigated by applying the Design of the Experiment in normal heptane isomerization. The design was guided to three surface responses on the dependency of normal heptane conversion, isomerization selectivity, and research octane number on the reaction temperature (200 – 350 °C), the weight percentage of protonated Zeolite Socony Mobil–5 (HZSM-5) zeolite in catalyst structure (10 – 40 weight percent) and time on stream (1 – 6 h). The Analysis of Variance for Pt-supported micro/mesoporous catalysts indicated that the reaction temperature was the prominent significant variable in normal heptane conversion, the development of isomers, and research octane number followed by weight percent of HZSM-5 and time on stream. The optimum research octane number predicted from the response surface analysis is ~ 61 at the reaction temperature of 350 °C, weight percent of HZSM-5 of 25%, and time on stream of 1 h.
Highlights
- In accordance with the obtained response, RSM can successfully predict the reaction rates.
- The highest octane number (~ 61) has occurred in the 25 wt% of HZSM-5, 350 °C and time on stream of 1 h.
- The reaction temperature is the most effective parameter on variable n-heptane conversion, the development of isomers, and research octane number.
- Other parameters, especially weight percent of HZSM-5 and then time on stream, have respectively effect on the responses.