Evaluation of electrocatalytic activity Ti and Al as anodes for the remediation of textile dyeing effluent
Authors
-
Asath Murphy Maria Stephen
 1
1
-
Jovitha Jane David
 1
1
-
RIJU S ROBIN
 1
1
-
Sahaya Leenus Sebastian
 1
1
- Jegathambal Palanichamy 2
-
Parameswari Kalivel
*
 1
1
Abstract
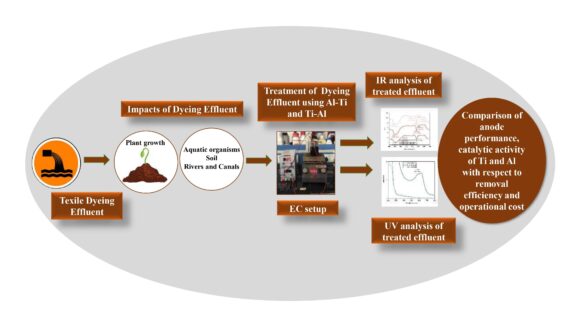
One of the industries that uses huge volumes of water is the textile sector, which generates a lot of
wastewaters. Electrocoagulation (EC), an environmentally friendly method, has been utilized to
remediate textile dyeing effluent. The goal of this work is to compare the catalytic activity of Ti
with Al using two sets of experiments (Al-Ti, Ti-Al), one of which contains Al as an anode and in
the other Ti as an anode to treat textile dyeing effluent to reduce operating costs of the process as
Ti electrode undergoes uniform dissolution with less energy consumption compared to Al. The
maximum color removal efficiency (CRE) of 97.13% and 96.8% was obtained while using Al-Ti
and Ti-Al electrodes. The removal efficiency of Total Dissolved Solids (TDS), Total Suspended
Solids (TSS), and Chemical Oxygen Demand (COD) were also found to be comparable with both
electrodes. The FTIR analysis of treated water demonstrated that the pollutant removal was similar
in both electrodes. The generation of hydroxides during the EC process is demonstrated by XPS
examination of sludge, with Al appearing as Al2p in the +3-oxidation state and Ti appearing as
Ti2p1/2 and Ti2p3/2 in the +4-oxidation state. The operating cost calculated for Al-Ti and Ti-Al
electrodes was found to be 2.90 US and 0.87 US. The difference in operating cost between these
two electrodes was found to be 70%. The energy, electrode consumption, and operating cost for
the Al-Ti electrode were found to be high due to its high dissolution.
Highlights
· The catalytic activity of Al and Ti as anode was compared in order to treat the textile dyeing effluent by Electrocoagulation process
· Optimization of operational parameters like pH, Voltage, Reaction time, Electrolyte concentration was done in order to get maximum removal efficiency
· Removal of pollutants after EC process was studied using FTIR analysis.
· Sludge obtained after EC process was subjected to XPS analysis to study the reaction mechanism.
· The results showed that Ti has better catalytic activity as anode treat textile dyeing effluent as the operational cost of the process is low while using Ti as anode compared to Al as anode.