Effectiveness of Ceria and Stania Nanoparticles in Photodegradation Tenoxicam Antibiotics Using UV-H2O2
Authors
Abstract
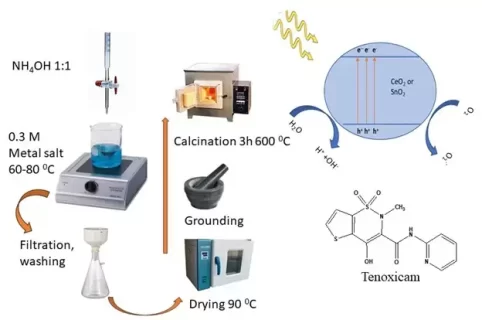
Both metal oxides, ceria (CeO2) and stania (SnO2) are prepared by precipitation technique using ammonia solution (1:1) and characterized by thermogravimetric analysis (TGA), x-ray powder diffraction (XRD), transmission electron microscope (TEM), nitrogen adsorption-desorption (for the specific surface area determination, SBET) and used in the photodegradation of Tenoxicam (TEN) antibiotics by using ultraviolet irradiation in presence of hydrogen peroxide. The obtained results showed that ceria and stania have a comparable surface area of 12 and 11 m2 g-1, respectively, and the crystallite size measured by XRD was found to be 44 and 18 nm for ceria and stania, respectively. Also, the results show that by increasing the exposure time, the amount of degraded antibiotic was increased. Data obtained show that both oxides, ceria, and stania can be used effectively as catalysts in the photodegrading process as photocatalysts. Stania is faster than ceria to degrade TEN antibiotics. Ceria needs more time than stania to degrade the drug at 100%, in which the TEN is fully degraded in the presence of stania and H2O2 at 40 minutes under certain conditions.