Diisopropyl Ether Production via Isopropanol Catalytic Dehydration over Zirconium Phosphate Modified Natural Zeolite
Authors
-
Hasanudin Hasanudin
*
 1
1
- Wan Ryan Asri 1, 2
-
Jeniva Rindi Anindia
 3, 2
3, 2
-
Suheryanto Suheryanto
 3, 2
3, 2
- Zainal Fanani 1, 2
-
Nino Rinaldi
 1
1
-
Muhammad Al Muttaqii
 1
1
Abstract
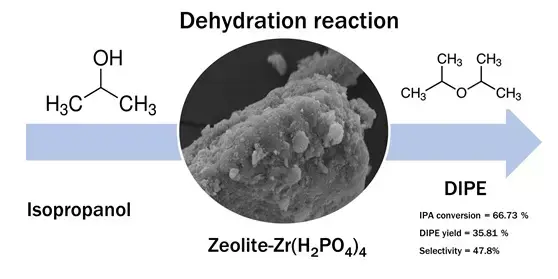
In this work, diisopropyl ether (DIPE) was produced through catalytic dehydration of isopropanol over zirconium phosphate-modified phosphate modified natural zeolite. The catalyst was prepared via the wet impregnation method. They were tested at 150 ˚C for 3 hours under a reflux system. The effect of zeolite-Zr(H2PO4)4 metal loading and zeolite-Zr without phosphate incorporation on dehydration isopropanol was also assessed. The results showed the natural zeolite was successfully modified as confirmed by XRD, FTIR, SEM-EDX, N2 physisorption, and catalyst acidity by the gravimetric technique. The highest isopropanol conversion (66.73%) was accomplished by 8 mEq/g zeolite-Zr(H2PO4)4 followed by the DIPE yield and selectivity up to 35.81% and 47.8%, respectively. Further reusability investigation showed that zeolite-Zr(H2PO4)4 catalyst provided adequate reusability up to the fourth reused with relatively decreased catalytic activity towards isopropanol dehydration.
Highlights
- The natural zeolite was modified via the wet impregnation method.
- The natural zeolite was successfully modified as confirmed by XRD, FTIR, SEM-EDX, N2 physisorption, and catalyst acidity measurements.
- The zeolite-Zr(H2PO4)4 had higher catalytic activity than zeolite-Zr towards isopropanol dehydration
- The zeolite-Zr(H2PO4)4 catalyst (8 mEq/g metal loading) exhibited the highest isopropanol conversion, DIPE yield and selectivity, respectively.
- Zeolite-Zr(H2PO4)4 catalyst provided adequate reusability up to the fourth reused towards isopropanol dehydration.