Application of Fe3O4/SiO2/CeO2 nanocomposite, an efficient and magnetic catalyst, to synthesize 2,3-dihydroquinazolin-4(1H)-ones derivatives
Authors
Abstract
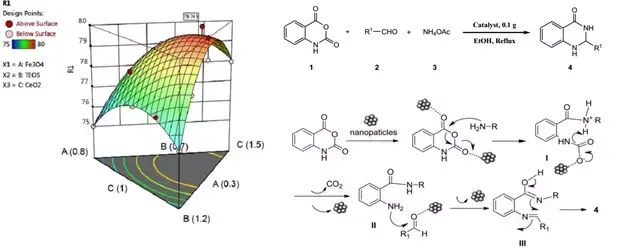
A supported magnetic nanocomposite as a simple, stable, and efficient catalyst was successfully developed for condensation reaction of aldehydes, ammonium acetate, and isatoic anhydride to prepare 2,3-dihydroquinazolin-4(1H)-one derivatives as essential biologically active heterocyclic compounds. Ethanol as a non-toxic solvent under a reflux condition was utilized in the reactions. The Fe3O4/SiO2/CeO2 nanocomposite was prepared as a magnetic and novel catalyst. The value of components of the catalyst composite, including Fe3O4, SiO2, and CeO2, was optimized using experimental design to prepare the best catalyst composite with the highest reaction efficiency. The optimum amounts of Fe3O4, SiO2, and CeO2 in the catalyst composite were 0.37 g, 0.85 mL, and 1.28 g, respectively. The catalyst structure was characterized by FT-IR spectroscopy, vibrating sample magnetometer, Powder X-ray diffraction, and Transmission electron microscope. A sol-gel procedure was utilized to prepare the catalyst, in which chemical bonds between the catalysis components, leading to a high chemical, mechanical, and thermal stability of the catalyst. Several syntheses of 2,3-dihydroquinazolin-4(1H)-ones derivatives were performed using Fe3O4/SiO2/CeO2 (0.1 g) in EtOH (10.0 mL) under reflux for 9-19 min with yield in the range of 89-97%. The method displayed various advantages, including high yields, easy workup, low catalyst consumption, high catalyst reusability, low reaction times, and fast and straightforward catalyst separation using a magnet.