Synthesis and characterization of Carbon-inserted phenolic resin nanocomposites
Authors
Abstract
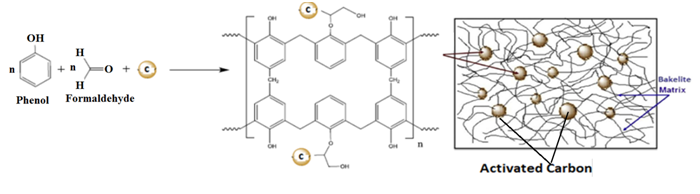
This paper presents the synthesis and characterization of nanocomposites made from Activated Carbon and Phenol-Formaldehyde, known for their exceptional thermal properties, chemical stability, and affinity for graphite and other forms of carbon. These composites are primarily designed for high-temperature applications that demand strength retention. X-ray diffraction (XRD) analysis reveals a distinct carbon peak in the nanocomposites, while Fourier-transform infrared (FTIR) spectroscopy indicates the presence of functional group peaks in their respective regions. The aim of this study is to provide a detailed account of the chemical synthesis and characterization of activated carbon/Phenol-Formaldehyde nanocomposites. The results of the XRD and FTIR analyses demonstrate the presence of a sharp carbon peak and functional group peaks in their respective regions. These properties render the composites suitable for high-temperature applications requiring strength retention.
Graphical Abstract
Keywords
References
- Liu L., Ruan R., Yang W. W., (2017), Clay/polymer nanocomposites for biomedical applications: A review. Mater. Sci. Eng. C. 75: 1324-1327.
- Amini Nia A., Pourshamsian Kh., Sadeghi B., (2020), Nano-ZnO impregnated on starch highly efficient heterogeneous bio-based catalyst for one-pot synthesis of pyranopyrimidinone and xanthene derivatives as potential. J. Org. Chem. 56: 1279-1288. https://doi.org/10.1134/S1070428020070234
- Osayemwenre G. O., Meyer E. L., (2014), Thermal decomposition of EVA composite encapsulant of single junction amorphous silicon photovoltaic (PV) module. J. Ovonic Res. 10: 221-225.
- Rezaei R., Darzi S. J., Yazdani M., (2020), Synthesis and Evaluation of 198Au/PAMAM-MPEG-FA against Cancer Cells. Antican. Agents in Medic. Chem. 20: 1250-1265. doi: 10.2174/1871520620666200220113452. PMID: 32077832. https://doi.org/10.2174/1871520620666200220113452
- Park S., Lee K. H., (2015), Polymer-metal nanocomposites: A review. J. Ind. Eng. Chem. 31: 1-6.
- Lin Y., Li D., Li X., Li G., Huang Z., (2014), Study on the mechanical properties of phenolic resin composites filled with carbon nanotubes. Polym. Compos. 35: 343-346. https://doi.org/10.1002/pc.22778
- Zhang M., Lai X., Xu Y., Cao J., (2017), Surface modification of alumina nanoparticles for improving the scratch resistance of phenolic resin-based composites. Compos. Part B: Eng. 125: 114-117.
- Fong H., Lau K. T., (2009), Effect of filler loading on phenolic resin composites. Electronic J. Mater. Sci. 44: 4659-4663.
- Fang X., Zhai T., Gautam U. K., Li L., Wu L., Bando Y., Golberg D., (2011), ZnS nanostructures: From synthesis to application. Prog. Mater. Sci. 56: 175-179. https://doi.org/10.1016/j.pmatsci.2010.10.001
- Thottoli A., Achuthanunni A., (2013), Effect of trisodium citrate concentration on the particle growth of ZnS nanoparticles. J. Nanostruc. Chem. 3: 56-60. https://doi.org/10.1186/2193-8865-3-56
- Kalu O., M., Amah A. N., Echi I., (2019), Physiochemical properties of mixed twin clay deposits in Awgbu used for pottery and possible structural applications. Niger. J. Technol. 38: 355-363. https://doi.org/10.4314/njt.v38i2.12
- Tiwary C. S., Kumbhakar P., Mitra A. K., Chattopadhyay K., (2009), Synthesis of wurtzite-phase ZnS nanocrystal and its optical properties. J. Lumines. 129: 1366-1369. https://doi.org/10.1016/j.jlumin.2009.07.004
- Kole A. K., Kumbhakar P., (2012), Cubic-to-hexagonal phase transition and optical properties of chemically synthesized ZnS nanocrystals. Res. Phys. 2: 150-155. https://doi.org/10.1016/j.rinp.2012.09.010
- Ummartyotin S., Bunnak N., Juntaro J., Sain M., Manuspiya H., (2012), Synthesis and luminescence properties of ZnS and metal (Mn, Cu)-doped-ZnS ceramic powder. Solid State Sci. 14: 299-303. https://doi.org/10.1016/j.solidstatesciences.2011.12.005
- Horoz S., Dai Q., Maloney F. S., Yakami B., Pikal J. M., Zhang X., Wang J., Wang W., Tang J., (2015), Absorption induced by Mn doping of ZnS for improved sensitized quantum-dot solar cells. Phys. Rev. Appl. 3: 1-7. https://doi.org/10.1103/PhysRevApplied.3.024011
- Sahare S., Dhoble S. J., Singh P., Ramrakhiani M., (2013), Fabrication of ZnS : Cu/PVA nanocomposite electroluminescence devices for flat panel displays. Adv. Mater. Lett. 4: 169-173. https://doi.org/10.5185/amlett.2012.6374
- Jayaseelan C., Ramkumar R., Rahuman A. A., Perumal P., (2013), Green synthesis of gold nanoparticles using seed aqueous extract of Abelmoschus esculentus and its antifungal activity. Indus. Crops and Products. 45: 423-429. https://doi.org/10.1016/j.indcrop.2012.12.019
- Vidya C., Hiremath S., Chandraprabha M. N., Antonyraj M. A. L., Gopala I. V., Jain A., Bansal K., (2013), Green synthesis of ZnO nanoparticles by calotropisgigantea. Int. J. Curr. Eng. Technol. 1: 118-120.
- Hudlikar M., Joglekar S., Dhaygude M., Kodama K., (2012), Latex-mediated synthesis of ZnS nanoparticles: green synthesis approach. J. Nanoparticle Res. 14: 865-870. https://doi.org/10.1007/s11051-012-0865-x
- Senapati U. S., Sarkar D., (2014), Characterization of biosynthesized zinc sulphide nanoparticles using edible mushroom Pleurotuss ostreatu. Indian J. Phys. 88: 557-562. https://doi.org/10.1007/s12648-014-0456-z
- Senapati U. S., Jha D. K., Sarkar D., (2013), Green synthesis and characterization of ZnS nanoparticles. Res. J. Phys. Sci. 1: 1-6.
- Tiwari A., Khan S. A., Kher R. S., Dhoble S. J., (2014), Synthesis, characterization and optical studies of highly luminescent ZnS nanoparticles associated with hypromellose matrix as a green and novel stabilizer. Lumines. 29: 637-641. https://doi.org/10.1002/bio.2597
- Zhang, M., Rong, M. Z., (2019), Nanocomposites based on carbon nanotubes and polymer matrices. Polym. Rev. 59: 1-5.
- Patton R. D., Pittman Jr. C. U., Wang L., Hill J. R., (1999), Vapor-grown carbon fiber composites with epoxy and poly(phenylene sulfide) matrices. Composites A. 30: 1081-1091. https://doi.org/10.1016/S1359-835X(99)00018-4
- Yu Q.-C., Wan H., (2012), Ablation capability of flake graphite reinforced barium-phenolic resin composite under long pulse laser irradiation. Wuji Cailiao Xuebao/J. Inorg. Mater. 27: 157-161. https://doi.org/10.3724/SP.J.1077.2012.00157
- Liu Y., Lu Z., Chen X., Wang D., Liu J., Hu L., (2009), Study on phenolic-resin/carbon-fibre ablation composites modified with polyhedral oligomeric silsesquioxanes. Proceed. 4th IEEE Int. Conf. Nano/Micro Eng. Molec. Sys. (pp. 605-608). Shenzhen, China.
- Bahramian A. R., Kokabi M., (2009), Ablation mechanism of polymer layered silicate nanocomposite heat shield. J. Hazard. Mater. 166: 445-454. https://doi.org/10.1016/j.jhazmat.2008.11.061
- Srikanth I., Daniel A., Kumar S., (2010), Nano silica-modified carbon-phenolic composites for enhanced ablation resistance. Scripta Mater. 63: 200-203. https://doi.org/10.1016/j.scriptamat.2010.03.052
- Koo J. H., Natali M., Tate J. S., Allcorn E., (2013), Polymer nanocomposites as ablative materials: A comprehensive review. Int. J. Energetic Mater. Chem. Propulsion. 12: 199-162. https://doi.org/10.1615/IntJEnergeticMaterialsChemProp.2013005383
- Natali M., Monti M., Kenny J., Torre L., (2011), Synthesis and thermal characterization of phenolic resin/silica nanocomposites prepared with high shear rate-mixing technique. J. Appl. Polym. Sci. 120: 2632-2640. https://doi.org/10.1002/app.33494
- Natali M., Monti M., Kenny J. M., Torre L., (2011), A nanostructured ablative bulk moulding compound: Development and characterisation. Composites A. 42: 1197-1204. https://doi.org/10.1016/j.compositesa.2011.04.022
- Natali M., Monti M., Puglia D., Kenny J. M., Torre L., (2012), Ablative properties of carbon black and MWNT/phenolic composites: A comparative study. Composites A. 43: 174-182. https://doi.org/10.1016/j.compositesa.2011.10.006
- Wang C., Cheng H., Hong C., Zhang X., Zeng T., (2018), Lightweight chopped carbon fibre reinforced silica-phenolic resin aerogel nanocomposite: Facile preparation, properties, and application to thermal protection. Compos. Part A: Appl. Sci. Manufac. 112: 81-90. https://doi.org/10.1016/j.compositesa.2018.05.026
- Zhang M., Rong M. Z., (2019), Nanoparticle-reinforced polymer nanocomposites. Polym. Rev. 59: 44-48.
- Taghiyari H. R., (2014), Effect of metal nanoparticles on hardness in particleboard. Int. J. Nano Dimens. 5: 379-386.
- Beiranvand M., Farhadi S., Mohammadi A., (2019), Graphene Oxide/Hydroxyapatite/Silver (rGO/HAP/Ag) nanocomposite: Synthesis, characterization, catalytic and antibacterial activity. Int. J. Nano Dimens. 10: 180-194.
- Sadeghi B., (2018), Controlled growth and characterisation of Ag/ZnO nano tetrapods for humidity sensing. Chem. High Throughput Screen. 21: 462-467. https://doi.org/10.2174/1386207321666180717120417
- Ghane M., Sadeghi B., Jafari A. R., Paknejhad A., (2010), Synthesis and characterization of a bi-Oxide nanoparticle ZnO/CuO by thermal decomposition. Int. J. Nano Dimens. 1: 33-40.
- Huang Y., Li D., Wang X., Yan L., Xiao Z., (2012), Carbon-nanotube-modified phenol-formaldehyde resins for use as high-performance adhesives. J. Mater. Sci. 47: 5416-5423.
- Cheng Y., Zhang Y., Yang L., Wang H., (2019), Quantum dot-based polymer nanocomposites for optoelectronic applications. Nanos. Res. Lett. 14: 28-33.
- Yang X., Zeng X., Han G., Sui D., Song X., Zhang Y., (2020), Preparation and performance of porous Carbon nanocomposite from renewable phenolic resin and halloysite nanotube. Nanomater. 10: 1703-1705. https://doi.org/10.3390/nano10091703
- Zhang M., Rong M. Z., (2019), Nanocomposites based on carbon nanotubes and polymer matrices. Polym. Rev. 59: 1-5.
- Maria A., (2019), Nanoparticle-reinforced polymer. Polymers. 11: 625-629. https://doi.org/10.3390/polym11040625
- Vella Durai S. C., Kumar E., Indiara R., Muthuraj D., (2020), Preparation and investigation of structural, optical, and conductivity properties of polyaniline dioxide nanocomposites. J. Ovonic Res. 16: 345-348. https://doi.org/10.15251/JOR.2020.166.345
- Sadeghi B., (2018), Synthesis and characterisation of ultrafine Ag/ZnO nanotetrapods (AZNTP) for environment humidity sensing. J. Env. Health Eng. 5: 115-119. https://doi.org/10.15171/ajehe.2018.15
- Bankar A., Joshi B., Ravi Kumar A., Zinjarde S., (2010), Banana peel extract mediated synthesis of gold nanoparticles. Colloids Surf. B. Biointerfaces. 80: 45-49. https://doi.org/10.1016/j.colsurfb.2010.05.029
- Bankar A., Joshi B., Ravi Kumar A., Zinjarde S., (2010), Banana peel extract mediated novel route for the synthesis of silver nanoparticles. Colloids Surf. A Physicochem. Eng. Asp., 368: 58-63. https://doi.org/10.1016/j.colsurfa.2010.07.024
- Zhou G. J., Li S. H., Zhang Y. C., Fu Y. Z., (2014), Biosynthesis of CdS nanoparticles in banana peel extract. J. Nanosc. Nanotechnol. 14: 4437-4442. https://doi.org/10.1166/jnn.2014.8259
- Happi Emaga T., Robert C., Ronkart S. N., Wathelet B., Paquot M., (2008), Dietary fibre components and pectin chemical features of peels during ripening in banana and plantain varieties. Biores. Technol. 99: 4346-4354. https://doi.org/10.1016/j.biortech.2007.08.030
- Verma D., Kole A. K., Kumbhakar P., (2015), Red shift of the band-edge photoluminescence emission and effects of annealing and capping agent on structural and optical properties of ZnO nanoparticles. J. Alloys and Comp. 625: 122-130. https://doi.org/10.1016/j.jallcom.2014.11.102
- Singh B., Chauhan N., (2009), Modification of psyllium polysaccharides for use in oral insulin delivery. Food Hydrocolloids. 23, 928-935. https://doi.org/10.1016/j.foodhyd.2008.06.004
- Basak P., Adhikari B., (2009), Poly (vinyl alcohol) hydrogels for pH dependent colon targeted drug delivery. J. Mater. Sci: Mater. Medic. 20: 137-146. https://doi.org/10.1007/s10856-008-3496-0
- Kumar K., Kaith B. S., Mittal H., (2010), Utilization of acrylamide and natural polysaccharide based polymeric networks in PH controlled released of 5-Amino salicylic acid. J. Chil. Chem. Soc. 55: 522-526. https://doi.org/10.4067/S0717-97072010000400025
- Xiong J., Xiong S., Guo Z., Yang M., Chen J., Fan H., (2012), Ultrasonic dispersion of nano TiC powders aided by tween 80 addition. Ceram. Int. 38: 1815-1821. https://doi.org/10.1016/j.ceramint.2011.10.004