Revolutionizing cancer treatment through nanoengineered photosensitizer formulations for advanced photodynamic therapy
Authors
-
Pragya Pallavi
 1
1
-
Karthick Harini
 1
1
-
Atanu Mahata
 2
2
-
Anbazhagan Thirumalai
 1
1
-
Koyeli Girigoswami
 1
1
-
Agnishwar Girigoswami
*
 1
1
Abstract
Photodynamic therapy (PDT) is an approved minimum-invasive therapeutic approach authorized for the clinical treatment of various types of cancer and antibiotic-resistant microbial disorders. During PDT, a photosensitizing compound known as a photosensitizer (PS) deliberately accumulates in tissues. The PS is activated when exposed to a specific wavelength of visible light, generating reactive oxygen species and causing tumor regression and cell death. PDT has the advantage of being low in systemic toxicity and selective in destroying tumors accessible to light, making it an attractive alternative to other conventional cancer treatments without affecting healthy cells. Despite the challenges of poor aqueous solubility and lack of selectivity associated with PS, PDT has shown promise by employing nanoformulations, enabling selective distribution and concentration in highly localized tumor regions. Centered on the utilization of nanoparticles and nanocarriers in PDT to mitigate treatment drawbacks, the study unveils the effectiveness of nanoformulated photosensitizing agents in tumor destruction. This reveals refined PDT strategies for overcoming limitations and propelling advancements in theranostic applications.
Graphical Abstract
Keywords
References
[1] Girigoswami K., Saini D., Girigoswami A., (2021), Extracellular matrix remodeling and development of cancer. Stem Cell Reviews and Reports. 17: 739-747. https://doi.org/10.1007/s12015-020-10070-1
[2] Pierce G. B., Damjanov I., (1998), The pathology of cancer. The biological basis of cancer. Cambridge university press, Cambridge. UK: 14-49.
[3] Watanabe A., (2008), Cancer metastases research. Nova Publishers.
[4] Jain B. P., Pandey S., (2022), Understanding Cancer: From Basics to Therapeutics. Academic Press.
[5] Zülch K. J., (2013), Brain tumors: Their biology and pathology. Springer-Verlag.
[6] Sakthi Devi R., Girigoswami A., Siddharth M., Girigoswami K., (2022), Applications of gold and silver nanoparticles in theranostics. Appl. Biochem. Biotechnol. 194: 4187-4219. https://doi.org/10.1007/s12010-022-03963-z
[7] Pourjafari M., Ghane M., Kaboosi H., Sadeghi B., Rezaei A., (2022), Antibacterial properties of Ag–Cu alloy nanoparticles against multidrug-resistant pseudomonas aeruginosa through inhibition of quorum sensing pathway and virulence-related genes. J. Biomed. Nanotechnol. 18: 1196-1204. https://doi.org/https://doi.org/10.1166/jbn.2022.3331
[8] Miller K. D., Nogueira L., Mariotto A. B., Rowland J. H., Yabroff K. R., Alfano C. M., Jemal A., Kramer J. L., Siegel R. L., (2019), Cancer treatment and survivorship statistics. CA: A Cancer J. Clinic. 69: 363-385. https://doi.org/https://doi.org/10.3322/caac.21565
[9] Sharmiladevi P., Girigoswami K., Haribabu V., Girigoswami A., (2021), Nano-enabled theranostics for cancer. Mater. Adv. 2: 2876-2891. https://doi.org/10.1039/D1MA00069A
[10] Lazer L. M., Sadhasivam B., Palaniyandi K., Muthuswamy T., Ramachandran I., Balakrishnan A., Pathak S., Narayan S., Ramalingam S., (2018), Chitosan-based nano-formulation enhances the anticancer efficacy of hesperetin. Int. J. Biol. Macromol. 107: 1988-1998. https://doi.org/https://doi.org/10.1016/j.ijbiomac.2017.10.064
[11] Nayagam V., Ponnambalam A., Michael Raj Lourdhurajan J., Samy A., Ignacimuthu S., (2023), Cytotoxic properties (MDA-MB-231- an epithelial breast cancer cell line) and bactericidal activity of Silver nanoparticles mediated by Strobilanthes ciliata nees. Int. J. Nano Dimens. 14: 356-365. https://doi.org/10.22034/ijnd.2023.1995048.2252
[12] Kuijpers D. I. M., Thissen M. R. T. M., Neumann M. H. A., (2002), Basal cell carcinoma. Am. J. Clinic. Dermat. 3: 247-259. https://doi.org/10.2165/00128071-200203040-00003
[13] Girigoswami A., Yassine W., Sharmiladevi P., Haribabu V., Girigoswami K., (2018), Camouflaged nanosilver with excitation wavelength dependent high quantum yield for targeted theranostic. Scientif. Rep. 8: 1-7. https://doi.org/10.1038/s41598-018-34843-4
[14] Sharmiladevi P., Akhtar N., Haribabu V., Girigoswami K., Chattopadhyay S., Girigoswami A., (2019), Excitation wavelength independent carbon-decorated ferrite nanodots for multimodal diagnosis and stimuli responsive therapy. ACS Appl. Bio Mater. 2: 1634-1642.
[15] Amininia A., Pourshamsian K., Sadeghi B., (2020), Nano-ZnO impregnated on Starch—A highly efficient heterogeneousbio-based catalyst for one-pot synthesis of pyranopyrimidinone and xanthene derivativesas potential antibacterial agents. Russ. J. Org. Chem. 56: 1279-1288. https://doi.org/10.1134/S1070428020070234
[16] Mehrgou A., Akouchekian M., (2016), The importance of BRCA1 and BRCA2 genes mutations in breast cancer development. Med. J. Islam. Repub. Iran. 30: 369-375.
[17] Mun H. y., Girigoswami A., Jung C., Cho D.-Y., Park H. G., (2009), SNPs detection by a single-strand specific nuclease on a PNA zip-code microarray. Biosens. Bioelect. 24: 1706-1711. https://doi.org/https://doi.org/10.1016/j.bios.2008.08.049
[18] Girigoswami A., Li T., Jung C., Mun H. Y., Park H. G., (2009), Gold nanoparticle-based label-free detection of BRCA1 mutations utilizing DNA ligation on DNA microarray. J. Nanosc. Nanotechnol. 9: 1019-1024. https://doi.org/10.1166/jnn.2009.C077
[19] Hodgson S. V., Maher E. R., Hodgson S. (1999), A practical guide to human cancer genetics (Vol. 2). Springer.
[20] Ryerson A. B., Eheman C., Styles T., Rycroft R., Snyder C., (2015), Connecting the dots: Linking the national program of cancer registries and the needs of survivors and clinicians. Am. J. Prev. Med. 49: S528-S535. https://doi.org/https://doi.org/10.1016/j.amepre.2015.08.026
[21] Zhu G., Cai H., Zheng Z., (2023), Cemiplimab combined with chemotherapy versus chemotherapy in advanced non-small cell lung cancer: an updated EMPOWER-Lung 3 trial-based cost-effectiveness analysis. Therap. Adv. Med. Onc. 15: 17588359231213619. https://doi.org/10.1177/17588359231213619
[22] Siegel R. L., Miller K. D., Wagle N. S., Jemal A., (2023), Cancer statistics, 2023. Ca. Cancer J. Clin. 73: 17-48.
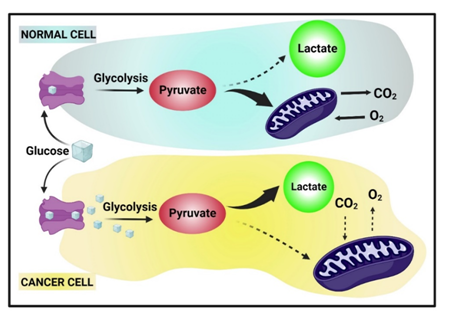
[23] Liberti M. V., Locasale J. W., (2016), The warburg effect: How does it benefit cancer cells? Trends Biochem. Sci. 41: 211-218. https://doi.org/10.1016/j.tibs.2015.12.001
[24] Girigoswami K., Pallavi P., Girigoswami A., (2023), Intricate subcellular journey of nanoparticles to the enigmatic domains of endoplasmic reticulum. Drug Delivery. 30: 2284684. https://doi.org/10.1080/10717544.2023.2284684
[25] Bellacosa A., Kumar C. C., Cristofano A. D., Testa J. R., (2005), Activation of AKT kinases in cancer: Implications for therapeutic targeting. In. Adv. Cancer Res. 94: 29-86. https://doi.org/https://doi.org/10.1016/S0065-230X(05)94002-5
[26] Hanahan D., Weinberg R. A., (2011), Hallmarks of cancer: The next generation. cell. 144: 646-674.
[27] Dranoff G., (2004), Cytokines in cancer pathogenesis and cancer therapy. Nature Rev. Cancer. 4: 11-22. https://doi.org/10.1038/nrc1252
[28] Perera Molligoda Arachchige A. S., (2021), Human NK cells: From development to effector functions. Innate Immunity. 27: 212-229. https://doi.org/10.1177/17534259211001512
[29] Amsaveni G., Farook A. S., Haribabu V., Murugesan R., Girigoswami A., (2013), Engineered multifunctional nanoparticles for DLA cancer cells targeting, sorting, MR imaging and drug delivery. Adv. Sci. Eng. Medic. 5: 1340-1348. https://doi.org/10.1166/asem.2013.1425
[30] Finn O. J., (2012), Immuno-oncology: Understanding the function and dysfunction of the immune system in cancer. Annals of Oncology. 23: viii6-viii9. https://doi.org/https://doi.org/10.1093/annonc/mds256
[31] Kavya J., Amsaveni G., Nagalakshmi M., Girigoswami K., Murugesan R., Girigoswami A., (2013), Silver nanoparticles induced lowering of BCl2/Bax causes Dalton's lymphoma tumour cell death in mice. J. Bionanosc. 7: 276-281. https://doi.org/10.1166/jbns.2013.1135
[32] Ghosh U., Nagalakshmi M., Sadhukhan R., Girigoswami A., (2013), Biocapped nanoparticles are more bioactive to heLa cells than its chemical counterpart. Adv. Sci. Eng. Medic. 5: 783-788. https://doi.org/10.1166/asem.2013.1359
[33] Kenter G. G., Welters M. J., Valentijn A. R. P., Lowik M. J., Berends-van der Meer D. M., Vloon A. P., Essahsah F., Fathers L. M., Offringa R., Drijfhout J. W., (2009), Vaccination against HPV-16 oncoproteins for vulvar intraepithelial neoplasia. New Engl. J. Medic. 361: 1838-1847.
[34] Chang M.-H., (2009), Cancer prevention by vaccination against hepatitis B. cancer prevention II, Berlin, Heidelberg.
[35] Girigoswami A., Girigoswami K., (2023), Potential applications of nanoparticles in improving the outcome of lung cancer treatment. Genes. 14: 1370-1376. https://www.mdpi.com/2073-4425/14/7/1370
[36] Ghosh S., Girigoswami K., Girigoswami A., (2019), Membrane-encapsulated camouflaged nanomedicines in drug delivery. Nanomedic. 14: 2067-2082.
[37] Schreiber R. D., Old L. J., Smyth M. J., (2011), Cancer immunoediting: Integrating immunity’s roles in cancer suppression and promotion. Science. 331: 1565-1570. https://doi.org/doi:10.1126/science.1203486
[38] Shurfa M. K., Girigoswami A., Sakthi Devi R., Harini K., Agraharam G., Deepika B., Pallavi P., Girigoswami K., (2023), Combinatorial effect of doxorubicin entrapped in alginate-chitosan hybrid polymer and cerium oxide nanocomposites on skin cancer management in mice. J. Pharmac.l Sci. 112: 2891-2900. https://doi.org/https://doi.org/10.1016/j.xphs.2023.08.014
[39] Janani G., Girigoswami A., Girigoswami K., (2023), Supremacy of nanoparticles in the therapy of chronic myelogenous leukemia. ADMET DMPK. 11: 499-511. https://doi.org/10.5599/admet.2013
[40] Pemula G., Anand A. V., Karthick H., Pragya P., Anbazhagan T., Koyeli G., Agnishwar G., (2023), Nanostructured proteins for delivering drugs to diseased tissues. Bioinsp. Biomimetic. Nanobiomater. 12: 115-129. https://doi.org/10.1680/jbibn.23.00004
[41] Gowtham P., Harini K., Thirumalai A., Pallavi P., Girigoswami K., Girigoswami A., (2023), Possible association of three polymorphisms in cytokine TNF-alpha (238G/A, 308G/A, 1031T/C) with polycystic ovary syndrome: A systematic review and meta-analysis. Biomedic. Res. Therap. 10: 5972-5986. https://doi.org/https://doi.org/10.15419/bmrat.v10i10.839
[42] Wilkie K. P., (2013), A review of mathematical models of cancer–immune interactions in the context of tumor dormancy. (Eds.), Systems Biology of Tumor Dormancy (pp. 201-234). Springer New York. https://doi.org/10.1007/978-1-4614-1445-2_10
[43] Trusolino L., Bertotti A., Comoglio P. M., (2010), MET signalling: Principles and functions in development, organ regeneration and cancer. Nature Rev. Molec. Cell Biol. 11: 834-848. https://doi.org/10.1038/nrm3012
[44] Yaswen P., MacKenzie K. L., Keith W. N., Hentosh P., Rodier F., Zhu J., Firestone G. L., Matheu A., Carnero A., Bilsland A., Sundin T., Honoki K., Fujii H., Georgakilas A. G., Amedei A., Amin A., Helferich B., Boosani C. S., Guha G., Ciriolo M. R., Chen S., Mohammed S. I., Azmi A. S., Bhakta D., Halicka D., Niccolai E., Aquilano K., Ashraf S. S., Nowsheen S., Yang X., (2015), Therapeutic targeting of replicative immortality. Seminars in Cancer Biology, 35: S104-S128. https://doi.org/https://doi.org/10.1016/j.semcancer.2015.03.007
[45] Kundu J. K., Surh Y.-J., (2008), Inflammation: Gearing the journey to cancer. Mutation Res./Rev. in Mutation Res. 659: 15-30. https://doi.org/https://doi.org/10.1016/j.mrrev.2008.03.002
[46] Grivennikov S. I., Greten F. R., Karin M., (2010), Immunity, inflammation, and cancer. Cell. 140: 883-899.
[47] Aggarwal B. B., Vijayalekshmi R. V., Sung B., (2009), Targeting inflammatory pathways for prevention and therapy of cancer: Short-term friend, long-term foe. Clin. Cancer Res. 15: 425-430. https://doi.org/10.1158/1078-0432.Ccr-08-0149
[48] Grivennikov S. I., Karin M., (2010), Inflammation and oncogenesis: A vicious connection. Current Opinion in Gen. Develop. 20: 65-71. https://doi.org/https://doi.org/10.1016/j.gde.2009.11.004
[49] Demaria S., Pikarsky E., Karin M., Coussens L. M., Chen Y. C., El-Omar E. M., Trinchieri G., Dubinett S. M., Mao J. T., Szabo E., Krieg A., Weiner G. J., Fox B. A., Coukos G., Wang E., Abraham R. T., Carbone M., Lotze M. T., (2010), Cancer and inflammation: Promise for biologic therapy. J. Immunother. 33: 335-351. https://doi.org/10.1097/CJI.0b013e3181d32e74
[50] Mantovani A., Allavena P., Sica A., Balkwill F., (2008), Cancer-related inflammation. Nature. 454: 436-444. https://doi.org/10.1038/nature07205
[51] Perwez Hussain S., Harris C. C., (2007), Inflammation and cancer: An ancient link with novel potentials. Int. J. Cancer. 121: 2373-2380. https://doi.org/https://doi.org/10.1002/ijc.23173
[52] Itzkowitz S. H., Yio X., (2004), Inflammation and cancer IV. colorectal cancer in inflammatory bowel disease: The role of inflammation. Am. J. Phys. Gastrointestinal Liver Physio. 287: G7-G17. https://doi.org/10.1152/ajpgi.00079.2004
[53] Macarthur M., Hold G. L., El-Omar E. M., (2004), Inflammation and cancer II. role of chronic inflammation and cytokine gene polymorphisms in the pathogenesis of gastrointestinal malignancy. Am. J. Physiol. Gastrointestinal Liver Physiol. 286: G515-G520. https://doi.org/10.1152/ajpgi.00475.2003
[54] Maisonneuve P., Lowenfels A. B., (2002), Chronic pancreatitis and pancreatic cancer. Dig Dis. 20: 32-37. https://doi.org/10.1159/000063165
[55] Baumgarten S. C., Frasor J., (2012), Minireview: Inflammation: An Instigator of more aggressive estrogen receptor (ER) positive breast cancers. Molec. Endocrinology. 26: 360-371. https://doi.org/10.1210/me.2011-1302
[56] Fouad T. M., Kogawa T., Reuben J. M., Ueno N. T., (2014), The role of inflammation in inflammatory breast cancer. (Eds.), Inflammation and Cancer (pp. 53-73). Springer Basel. https://doi.org/10.1007/978-3-0348-0837-8_3
[57] Risch H. A., Howe G. R., (1995), Pelvic inflammatory disease and the risk of epithelial ovarian cancer. Cancer Epidemiol. Biomarkers & Prevention. 4: 447-451.
[58] Harini K., Pallavi P., Gowtham P., Girigoswami K., Girigoswami A., (2022), Smart polymer-based reduction responsive therapeutic delivery to cancer cells. Current Pharmac. Rep. 8: 205-211. https://doi.org/10.1007/s40495-022-00282-z
[59] Rich J. N., Bao S., (2007), Chemotherapy and cancer stem cells. Cell stem cell. 1: 353-355.
[60] Pathak S., Zajac K. K., Annaji M., Govindarajulu M., Nadar R. M., Bowen D., Babu R. J., Dhanasekaran M., (2023), Clinical outcomes of chemotherapy in cancer patients with different ethnicities. Cancer Rep. 6: e1830. https://doi.org/https://doi.org/10.1002/cnr2.1830
[61] O'Donnell J. S., Hoefsmit E. P., Smyth M. J., Blank C. U., Teng M. W. L., (2019), The promise of neoadjuvant immunotherapy and surgery for cancer treatment. Clinic. Cancer Res. 25: 5743-5751. https://doi.org/10.1158/1078-0432.Ccr-18-2641
[62] Chu E., Sartorelli A. C., Katzung B., Masters S., Trevor A., (2007), Basic and clinical pharmacology. Cancer Chemoth. 10th ed. Boston: McGraw-Hill: 878-907.
[63] Chu E., Sartorelli A., (2018), Cancer chemotherapy. Lange’s Basic and Clinical Pharmacology: 948-976.
[64] An J., Peng C., Tang H., Liu X., Peng F., (2021), New advances in the research of resistance to neoadjuvant chemotherapy in breast cancer. Int. J. Molec. Sci. 22: 25-31.
[65] Tivnan A., Heilinger T., Lavelle E. C., Prehn J. H. M., (2017), Advances in immunotherapy for the treatment of glioblastoma. J. Neuro-Oncology. 131: 1-9. https://doi.org/10.1007/s11060-016-2299-2
[66] Xu Y., Xiong J., Sun X., Gao H., (2022), Targeted nanomedicines remodeling immunosuppressive tumor microenvironment for enhanced cancer immunotherapy. Acta Pharmac. Sinica B. 12: 4327-4347. https://doi.org/https://doi.org/10.1016/j.apsb.2022.11.001
[67] Finn O. J., (2008), Cancer immunology. New Engl. J. Medic. 358: 2704-2715.
[68] Mishra J., Drummond J., Quazi S. H., Karanki S. S., Shaw J. J., Chen B., Kumar N., (2013), Prospective of colon cancer treatments and scope for combinatorial approach to enhanced cancer cell apoptosis. Crit. Rev. Oncology/Hematology. 86: 232-250. https://doi.org/https://doi.org/10.1016/j.critrevonc.2012.09.014
[69] Kinker G. S., Vitiello G. A. F., Ferreira W. A. S., Chaves A. S., Cordeiro de Lima V. C., Medina T. d. S., (2021), B cell orchestration of anti-tumor immune responses: A matter of cell localization and communication [Review]. Front. Cell Develop.l Bio. 9: 67-78 https://doi.org/10.3389/fcell.2021.678127
[70] Callahan M. K., Wolchok J. D., Allison J. P., (2010), Anti–CTLA-4 antibody therapy: Immune monitoring during clinical development of a novel immunotherapy. Seminars in Oncol. 37: 473-484. https://doi.org/https://doi.org/10.1053/j.seminoncol.2010.09.001
[71] Lollini P.-L., Cavallo F., Nanni P., Forni G., (2006), Vaccines for tumour prevention. Nature Rev. Cancer. 6: 204-216. https://doi.org/10.1038/nrc1815
[72] Malik L. A., Bashir A., Qureashi A., Pandith A. H., (2019), Detection and removal of heavy metal ions: a review. Environm. Chem. Lett. 17: 1495-1521.
[73] Baskar R., Lee K. A., Yeo R., Yeoh K. W., (2012), Cancer and radiation therapy: Current advances and future directions. Int. J. Med. Sci. 9: 193-199. https://doi.org/10.7150/ijms.3635
[74] Wang K., Tepper J. E., (2021), Radiation therapy-associated toxicity: Etiology, management, and prevention. CA: A Cancer J. Clinic. 71: 437-454. https://doi.org/https://doi.org/10.3322/caac.21689
[75] Zeman E. M., Schreiber E. C., Tepper J. E., (2020), 27 - basics of radiation therapy. In J. E. Niederhuber, et al. (Eds.), Abeloff's Clinical Oncology (Sixth Edition) (pp. 431-460.e433). Elsevier. https://doi.org/https://doi.org/10.1016/B978-0-323-47674-4.00027-X
[76] Sagar J., Chaib B., Sales K., Winslet M., Seifalian A., (2007), Role of stem cells in cancer therapy and cancer stem cells: A review. Cancer Cell Internat. 7(1): 9-13. https://doi.org/10.1186/1475-2867-7-9
[77] Bagó J. R., Sheets K. T., Hingtgen S. D., (2016), Neural stem cell therapy for cancer. Methods. 99: 37-43. https://doi.org/https://doi.org/10.1016/j.ymeth.2015.08.013
[78] Girigoswami K., Devender N. S., Girigoswami A., (2021), Fate of stem cells grown on the extracellular matrix isolated from cancer cells and their possible applications in tissue engineering. Current Sci. 120: 1616-1622.
[79] Abbas Z., Rehman S., (2018), An overview of cancer treatment modalities. Neoplasm. 1: 139-157.
[80] Olson M. T., Ly Q. P., Mohs A. M., (2019), Fluorescence guidance in surgical oncology: Challenges, opportunities, and translation. Molec. Imag. Biol. 21: 200-218. https://doi.org/10.1007/s11307-018-1239-2
[81] Cianchi F., Staderini F., Badii B., (2014), Single-incision laparoscopic colorectal surgery for cancer: State of art. World J. Gastroenterol. 20: 6073-6080. https://doi.org/10.3748/wjg.v20.i20.6073
[82] Wyld L., Audisio R. A., Poston G. J., (2015), The evolution of cancer surgery and future perspectives. Nature Rev. Clinic. Oncol. 12: 115-124. https://doi.org/10.1038/nrclinonc.2014.191
[83] Yildizhan H., Barkan N. P., Karahisar Turan S., Demiralp Ö., Özel Demiralp F. D., Uslu B., Ōzkan S. A., (2018), Chapter 1 - Treatment strategies in cancer from past to present. In A. M. Grumezescu (Ed.), Drug Targeting and Stimuli Sensitive Drug Delivery Systems (pp. 1-37). William Andrew Publishing. https://doi.org/https://doi.org/10.1016/B978-0-12-813689-8.00001-X
[84] Husain R. S. A., Ramakrishnan V., (2015), Global variation of human papillomavirus genotypes and selected genes involved in cervical malignancies. Annals of Global Health. 81: 675-683. https://doi.org/https://doi.org/10.1016/j.aogh.2015.08.026
[85] Misra S., (2013), Human gene therapy: A brief overview of the genetic revolution. J. Assoc. Physicians India. 61: 127-133.
[86] Izquierdo M., (2005), Short interfering RNAs as a tool for cancer gene therapy. Cancer Gene Therapy. 12: 217-227. https://doi.org/10.1038/sj.cgt.7700791
[87] Alard E., Butnariu A.-B., Grillo M., Kirkham C., Zinovkin D. A., Newnham L., Macciochi J., Pranjol M. Z. I., (2020), Advances in anti-cancer immunotherapy: Car-T cell, checkpoint inhibitors, dendritic cell vaccines, and oncolytic viruses, and emerging cellular and molecular targets. Cancers. 12: 1826-1831. https://www.mdpi.com/2072-6694/12/7/1826
[88] Anand U., Dey A., Chandel A. K. S., Sanyal R., Mishra A., Pandey D. K., De Falco V., Upadhyay A., Kandimalla R., Chaudhary A., Dhanjal J. K., Dewanjee S., Vallamkondu J., Pérez de la Lastra J. M., (2023), Cancer chemotherapy and beyond: Current status, drug candidates, associated risks and progress in targeted therapeutics. Genes & Diseases. 10: 1367-1401. https://doi.org/https://doi.org/10.1016/j.gendis.2022.02.007
[89] Chen Y., Jungsuwadee P., Vore M., Butterfield D. A., St Clair D. K., (2007), Collateral damage in cancer chemotherapy: Oxidative stress in nontargeted tissues. Molec. Intervent. 7: 147-152.
[90] Nurgali K., Jagoe R. T., Abalo R., (2018), Adverse effects of cancer chemotherapy: Anything new to improve tolerance and reduce sequelae? In (Vol. 9, pp. 245): Frontiers Media SA.
[91] Majeed H., Gupta V., (2022), Adverse effects of radiation therapy. StatPearls Publishing, Treasure Island (FL). http://europepmc.org/books/NBK563259
[92] Koontz B. F., (2017), Radiation therapy treatment effects: An evidence-based guide to managing toxicity. Springer publishing company.
[93] Amer M. H., (2014), Gene therapy for cancer: Present status and future perspective. Molec. Cellul. Therap. 2: 27-32. https://doi.org/10.1186/2052-8426-2-27
[94] Tohme S., Simmons R. L., Tsung A., (2017), Surgery for cancer: A trigger for metastases. Cancer Res. 77: 1548-1552. https://doi.org/10.1158/0008-5472.Can-16-1536
[95] Chen J., Fan T., Xie Z., Zeng Q., Xue P., Zheng T., Chen Y., Luo X., Zhang H., (2020), Advances in nanomaterials for photodynamic therapy applications: Status and challenges. Biomater. 237: 119827. https://doi.org/https://doi.org/10.1016/j.biomaterials.2020.119827
[96] Castano A. P., Demidova T. N., Hamblin M. R., (2004), Mechanisms in photodynamic therapy: Part one—photosensitizers, photochemistry and cellular localization. Photodiag. Photodyn. Therapy. 1: 279-293. https://doi.org/https://doi.org/10.1016/S1572-1000(05)00007-4
[97] Filatov M. A., Senge M. O., (2016), Molecular devices based on reversible singlet oxygen binding in optical and photomedical applications. Molec. Sys. Des. Eng. 1: 258-272.
[98] Allison R. R., Moghissi K., (2013), Photodynamic therapy (PDT): PDT mechanisms. Clinic. Endoscopy. 46: 24-29.
[99] Robertson C. A., Evans D. H., Abrahamse H., (2009), Photodynamic therapy (PDT): A short review on cellular mechanisms and cancer research applications for PDT. J. Photochem. Photobiol. B: Biol. 96: 1-8. https://doi.org/https://doi.org/10.1016/j.jphotobiol.2009.04.001
[100] Dąbrowski J. M., Pucelik B., Regiel-Futyra A., Brindell M., Mazuryk O., Kyzioł A., Stochel G., Macyk W., Arnaut L. G., (2016), Engineering of relevant photodynamic processes through structural modifications of metallotetrapyrrolic photosensitizers. Coord. Chem. Rev. 325: 67-101. https://doi.org/https://doi.org/10.1016/j.ccr.2016.06.007
[101] Itoh T., (2012), Fluorescence and phosphorescence from higher excited states of organic molecules. Chem. Rev. 112: 4541-4568. https://doi.org/10.1021/cr200166m
[102] Xie J., Wang Y., Choi W., Jangili P., Ge Y., Xu Y., Kang J., Liu L., Zhang B., Xie Z., He J., Xie N., Nie G., Zhang H., Kim J. S., (2021), Overcoming barriers in photodynamic therapy harnessing nano-formulation strategies [10.1039/D0CS01370F]. Chem. Soc. Rev. 50: 9152-9201. https://doi.org/10.1039/D0CS01370F
[103] Housel J. P., Zeitouni N. C., (2011), Photodynamic therapy for nonmelanoma skin cancer. Cutaneous Malignancy of the Head and Neck: A Multidiscip. Approach. 205: 122-127.
[104] Agostinis P., Berg K., Cengel K. A., Foster T. H., Girotti A. W., Gollnick S. O., Hahn S. M., Hamblin M. R., Juzeniene A., Kessel D., (2011), Photodynamic therapy of cancer: An update. CA: A Cancer J. Clinic. 61: 250-281.
[105] Yoon I., Li J. Z., Shim Y. K., (2013), Advance in photosensitizers and light delivery for photodynamic therapy. CE. 46: 7-23. https://doi.org/10.5946/ce.2013.46.1.7
[106] Gunaydin G., Gedik M. E., Ayan S., (2021), Photodynamic therapy—current limitations and novel approaches [Review]. Front. Chem. 9: 21-27. https://doi.org/10.3389/fchem.2021.691697
[107] Hamblin M. R., Hasan T., (2004), Photodynamic therapy: A new antimicrobial approach to infectious disease? [10.1039/B311900A]. Photochem. Photobiol. Sci. 3: 436-450. https://doi.org/10.1039/B311900A
[108] Mallidi S., Anbil S., Bulin A.-L., Obaid G., Ichikawa M., Hasan T., (2016), Beyond the barriers of light penetration: Strategies, perspectives and possibilities for photodynamic therapy. Theranostics. 6: 2458-2463.
[109] Son J., Yi G., Yoo J., Park C., Koo H., Choi H. S., (2019), Light-responsive nanomedicine for biophotonic imaging and targeted therapy. Adv. Drug Deliv. Rev. 138: 133-147. https://doi.org/https://doi.org/10.1016/j.addr.2018.10.002
[110] Stringasci M. D., Fortunato T. C., Moriyama L. T., Filho J. D. V., Bagnato V. S., Kurachi C., (2017), Interstitial PDT using diffuser fiber—investigation in phantom and in vivo models. Lasers in Medic. Sci. 32: 1009-1016. https://doi.org/10.1007/s10103-017-2225-7
[111] Kwiatkowski S., Knap B., Przystupski D., Saczko J., Kędzierska E., Knap-Czop K., Kotlińska J., Michel O., Kotowski K., Kulbacka J., (2018), Photodynamic therapy – mechanisms, photosensitizers and combinations. Biomedic. Pharmacoth. 106: 1098-1107. https://doi.org/https://doi.org/10.1016/j.biopha.2018.07.049
[112] Karimnia V., Slack F. J., Celli J. P., (2021), Photodynamic therapy for pancreatic ductal adenocarcinoma. Cancers. 13: 4354-4358. https://www.mdpi.com/2072-6694/13/17/4354
[113] McFarland S. A., Mandel A., Dumoulin-White R., Gasser G., (2020), Metal-based photosensitizers for photodynamic therapy: The future of multimodal oncology? Curr. Opin. Chem. Biol. 56: 23-27. https://doi.org/10.1016/j.cbpa.2019.10.004
[114] Simões J. C. S., Sarpaki S., Papadimitroulas P., Therrien B., Loudos G., (2020), Conjugated photosensitizers for imaging and PDT in cancer research. J. Medic. Chem. 63: 14119-14150. https://doi.org/10.1021/acs.jmedchem.0c00047
[115] Lismont M., Dreesen L., Wuttke S., (2017), Metal-organic framework nanoparticles in photodynamic therapy: Current status and perspectives. Adv. Funct. Mater. 27: 1606314. https://doi.org/https://doi.org/10.1002/adfm.201606314
[116] Kumar S. S., Harikrishnan K. K., Urmila S. P., Gauri V., Saritha A., Gangopadhyay M., (2023), Comprehensive review of Pluronic® polymers of different shapes with prominent applications in photodynamic therapy. Europ. Polym. J. 200: 112534. https://doi.org/https://doi.org/10.1016/j.eurpolymj.2023.112534
[117] Wachowska M., Muchowicz A., Firczuk M., Gabrysiak M., Winiarska M., Wańczyk M., Bojarczuk K., Golab J., (2011), Aminolevulinic acid (ALA) as a prodrug in photodynamic therapy of cancer. Molec. 16: 4140-4164. https://www.mdpi.com/1420-3049/16/5/4140
[118] Weijer R., Broekgaarden M., Kos M., van Vught R., Rauws E. A. J., Breukink E., van Gulik T. M., Storm G., Heger M., (2015), Enhancing photodynamic therapy of refractory solid cancers: Combining second-generation photosensitizers with multi-targeted liposomal delivery. J. Photochem. Photobiol. C: Photochem. Rev. 23: 103-131. https://doi.org/https://doi.org/10.1016/j.jphotochemrev.2015.05.002
[119] Lucky S. S., Soo K. C., Zhang Y., (2015), Nanoparticles in photodynamic therapy. Chem. Rev. 115: 1990-1995.
[120] Hua Z., (1996), Effectiveness of delta-aminolevulinic acid-induced protoporphyrin as a photosensitizer for photodynamic therapy in vivo and in vitro. University of Rochester.
[121] Janas K., Boniewska-Bernacka E., Dyrda G., Słota R., (2021), Porphyrin and phthalocyanine photosensitizers designed for targeted photodynamic therapy of colorectal cancer. Bioorg. Med. Chem. 30: 115926. https://doi.org/10.1016/j.bmc.2020.115926
[122] Zhou Z., Song J., Nie L., Chen X., (2016), Reactive oxygen species generating systems meeting challenges of photodynamic cancer therapy [10.1039/C6CS00271D]. Chem. Soc. Rev. 45: 6597-6626. https://doi.org/10.1039/C6CS00271D
[123] Mfouo-Tynga I. S., Dias L. D., Inada N. M., Kurachi, C., (2021), Features of third generation photosensitizers used in anticancer photodynamic therapy: Review. Photodiag. Photodynam. Therapy. 34: 102091-102096. https://doi.org/https://doi.org/10.1016/j.pdpdt.2020.102091
[124] Gierlich P., Mata A. I., Donohoe C., Brito R. M. M., Senge M. O., Gomes-da-Silva L. C., (2020), Ligand-targeted delivery of photosensitizers for cancer treatment. Molec. 25: 5317-5321. https://www.mdpi.com/1420-3049/25/22/5317
[125] Pallavi P., Girigoswami A., Girigoswami K., Hansda S., Ghosh R., (2022), Photodynamic therapy in cancer. In S. Chakraborti (Ed.), Handbook of Oxid. Stress in Cancer: Therap. Asp. (pp. 1285-1308). Springer Nature Singapore. https://doi.org/10.1007/978-981-16-5422-0_232
[126] Algorri J. F., Ochoa M., Roldán-Varona P., Rodríguez-Cobo L., López-Higuera J. M., (2021), Photodynamic therapy: A compendium of latest reviews. Cancers. 13: 4447-4452. https://www.mdpi.com/2072-6694/13/17/4447
[127] Mehraban N., Freeman H. S., (2015), Developments in PDT sensitizers for increased selectivity and singlet oxygen production. Mater. 8: 4421-4456. https://www.mdpi.com/1996-1944/8/7/4421
[128] Dąbrowski J. M., Arnaut L. G., (2015), Photodynamic therapy (PDT) of cancer: From local to systemic treatment [10.1039/C5PP00132C]. Photochem. Photobiol. Sci. 14: 1765-1780. https://doi.org/10.1039/C5PP00132C
[129] Konan Y. N., Gurny R., Allémann E., (2002), State of the art in the delivery of photosensitizers for photodynamic therapy. J. Photochem. Photobiol. B: Biology. 66: 89-106. https://doi.org/https://doi.org/10.1016/S1011-1344(01)00267-6
[130] Alsaab H. O., Alghamdi M. S., Alotaibi A. S., Alzhrani R., Alwuthaynani F., Althobaiti Y. S., Almalki A. H., Sau S., Iyer A. K., (2020), Progress in clinical trials of photodynamic therapy for solid tumors and the role of nanomedicine. Cancers. 12: 2793-2797. https://www.mdpi.com/2072-6694/12/10/2793
[131] Hu J., Tang Y. a., Elmenoufy A. H., Xu H., Cheng Z., Yang X., (2015), Nanocomposite-based photodynamic therapy strategies for deep tumor treatment. Small. 11: 5860-5887. https://doi.org/https://doi.org/10.1002/smll.201501923
[132] Lange C., Bednarski P., (2016), Photosensitizers for photodynamic therapy: Photochemistry in the service of oncology. Current Pharmac. Design. 22: 6956-6974.
[133] Abrahamse H., Hamblin M. R., (2016), New photosensitizers for photodynamic therapy. Biochem. J. 473: 347-352.
[134] Xie Z., Fan T., An J., Choi W., Duo Y., Ge Y., Zhang B., Nie G., Xie N., Zheng T., Chen Y., Zhang H., Kim J. S., (2020), Emerging combination strategies with phototherapy in cancer nanomedicine [10.1039/D0CS00215A]. Chem. Soc. Rev. 49: 8065-8087. https://doi.org/10.1039/D0CS00215A
[135] Kruger C. A., Abrahamse H., (2018), Utilisation of targeted nanoparticle photosensitiser drug delivery systems for the enhancement of photodynamic therapy. Molecules. 23: 2628-2632. https://www.mdpi.com/1420-3049/23/10/2628
[136] Liu D., Yang F., Xiong F., Gu N., (2016), The smart drug delivery system and its clinical potential. Theranostics. 6: 1306-1311.
[137] Ps S. S., Guha A., Deepika B., Udayakumar S., Nag M., Lahiri D., Girigoswami A., Girigoswami K., (2023), Nanocargos designed with synthetic and natural polymers for ovarian cancer management. Naunyn-Schmiedeberg's Archives of Pharmac. 396: 3407-3415. https://doi.org/10.1007/s00210-023-02608-0
[138] Pallavi P., Sharmiladevi P., Haribabu V., Girigoswami K., Girigoswami A., (2022), A nano approach to formulate photosensitizers for photodynamic therapy. Curr. Nanos. 18: 675-689. https://doi.org/10.2174/1573413718666211222162041
[139] Deepika R., Girigoswami K., Murugesan R., Girigoswami A., (2018), Influence of divalent cation on morphology and drug delivery efficiency of mixed polymer nanoparticles. Curr. Drug Deliv. 15: 652-657.
[140] Petroni S., Tagliaro I., Antonini C., D’Arienzo M., Orsini S. F., Mano J. F., Brancato V., Borges J., Cipolla L., (2023), Chitosan-based biomaterials: Insights into chemistry, properties, devices, and their biomedical applications. Marine Drugs. 21: 147-153. https://www.mdpi.com/1660-3397/21/3/147
[141] Šošić L., Selbo P. K., Kotkowska Z. K., Kündig T. M., Høgset A., Johansen P., (2020), Photochemical internalization: Light paves way for new cancer chemotherapies and vaccines. Cancers. 12: 165-171. https://www.mdpi.com/2072-6694/12/1/165
[142] Zhang C., Qin W.-J., Bai X.-F., Zhang X.-Z., (2020), Nanomaterials to relieve tumor hypoxia for enhanced photodynamic therapy. Nano Today. 35: 100960-100964. https://doi.org/https://doi.org/10.1016/j.nantod.2020.100960
[143] Pawar B., Vasdev N., Gupta T., Mhatre M., More A., Anup N., Tekade R., (2022), Current update on transcellular brain drug delivery. Pharmaceutics 2022, 14, 2719. In: s Note: MDPI stays neutral with regard to jurisdictional claims in published ….
[144] Kumari A., Yadav S. K., Yadav S. C., (2010), Biodegradable polymeric nanoparticles based drug delivery systems. Colloids and Surf. B: Biointerf. 75: 1-18. https://doi.org/https://doi.org/10.1016/j.colsurfb.2009.09.001
[145] Debele T. A., Peng S., Tsai H.-C., (2015), Drug carrier for photodynamic cancer therapy. Int. J. Molec. Sci. 16: 22094-22136. https://www.mdpi.com/1422-0067/16/9/22094
[146] Murugan A., Sugumaran P. J., Ravikumar C. K. R. P., Raman N., Yadav H. S., Arasu P. T., (2022), Porphysomes and porphyrin-based nanomaterials for drug delivery system. In H. Barabadi, et al. (Eds.), Pharmac. Nanobiotechnol. Targeted Therapy (pp. 281-312). Springer International Publishing. https://doi.org/10.1007/978-3-031-12658-1_10
[147] Tedesco A. C., Primo F. L., Petrilli R., (2021), Nanosystems comprising biocompatible polymers for the delivery of photoactive compounds in biomedical applications. In J. O. Eloy, et al. (Eds.), Nanocarr. Drug Delivery: Concepts and Applic. (pp. 253-287). Springer International Publishing. https://doi.org/10.1007/978-3-030-63389-9_11
[148] Oliveira J. M., Salgado A. J., Sousa N., Mano J. F., Reis R. L., (2010), Dendrimers and derivatives as a potential therapeutic tool in regenerative medicine strategies—A review. Prog. Polymer Science. 35: 1163-1194. https://doi.org/https://doi.org/10.1016/j.progpolymsci.2010.04.006
[149] Singla P., Garg S., McClements J., Jamieson O., Peeters M., Mahajan R. K., (2022), Advances in the therapeutic delivery and applications of functionalized Pluronics: A critical review. Adv. Colloid and Interf. Sci. 299: 102563. https://doi.org/https://doi.org/10.1016/j.cis.2021.102563
[150] Wang Y.-W., Descalzo A. B., Shen Z., You X.-Z., Rurack K., (2010), Dihydronaphthalene-fused boron–dipyrromethene (BODIPY) dyes: Insight into the electronic and conformational tuning modes of BODIPY fluorophores. Chem. – A. Europ. J. 16: 2887-2903. https://doi.org/https://doi.org/10.1002/chem.200902527
[151] Castillo R. R., Lozano D., González B., Manzano M., Izquierdo-Barba I., Vallet-Regí M., (2019), Advances in mesoporous silica nanoparticles for targeted stimuli-responsive drug delivery: An update. Expert Opin. on Drug Del. 16: 415-439. https://doi.org/10.1080/17425247.2019.1598375
[152] Bayir S., Barras A., Boukherroub R., Szunerits S., Raehm L., Richeter S., Durand J.-O., (2018), Mesoporous silica nanoparticles in recent photodynamic therapy applications. Photochem. Photobiolog. Sci. 17: 1651-1674. https://doi.org/10.1039/c8pp00143j
[153] Tan K. F., In L. L. A., Vijayaraj Kumar P., (2023), Surface functionalization of Gold nanoparticles for targeting the tumor microenvironment to improve antitumor efficiency. ACS Appl. Bio Mater. 6: 2944-2981. https://doi.org/10.1021/acsabm.3c00202
[154] Lin L., Song X., Dong X., Li B., (2021), Nano-photosensitizers for enhanced photodynamic therapy. Photodiag. Photodyn. Therapy. 36: 102597. https://doi.org/https://doi.org/10.1016/j.pdpdt.2021.102597
[155] Garcia Calavia P., Bruce G., Perez-Garcia L., Russell D. A., (2018), Photosensitiser-gold nanoparticle conjugates for photodynamic therapy of cancer. Photochem. Photobiol. Sci. 17: 1534-1539.
[156] Huang P., Li Z., Lin J., Yang D., Gao G., Xu C., Bao L., Zhang C., Wang K., Song H., Hu H., Cui D., (2011), Photosensitizer-conjugated magnetic nanoparticles for in vivo simultaneous magnetofluorescent imaging and targeting therapy. Biomater. 32: 3447-3458. https://doi.org/10.1016/j.biomaterials.2011.01.032
[157] Wu Q., Chen G., Gong K., Wang J., Ge X., Liu X., Guo S., Wang F., (2019), MnO2-laden black phosphorus for MRI-guided synergistic PDT, PTT, and chemotherapy. Matter. 1: 496-512.
[158] Kashef N., Huang Y.-Y., Hamblin M. R., (2017), Advances in antimicrobial photodynamic inactivation at the nanoscale. Nanophotonics. 6: 853-879. https://doi.org/doi:10.1515/nanoph-2016-0189
[159] Shi J., Yu X., Wang L., Liu Y., Gao J., Zhang J., Ma R., Liu R., Zhang Z., (2013), PEGylated fullerene/iron oxide nanocomposites for photodynamic therapy, targeted drug delivery and MR imaging. Biomaterials. 34: 9666-9677. https://doi.org/https://doi.org/10.1016/j.biomaterials.2013.08.049
[160] Ramírez-García G., De la Rosa E., López-Luke T., Panikar S. S., Salas P., (2019), Controlling trapping states on selective theranostic core@shell (NaYF4:Yb,Tm@TiO2-ZrO2) nanocomplexes for enhanced NIR-activated photodynamic therapy against breast cancer cells [10.1039/C9DT00482C]. Dalton Transact. 48: 9962-9973. https://doi.org/10.1039/C9DT00482C
[161] Li J., Guo D., Wang X., Wang H., Jiang H., Chen B., (2010), The photodynamic effect of different size ZnO nanoparticles on cancer cell proliferation in vitro. Nanoscale Res. Lett. 5: 1063-1067. https://doi.org/10.1007/s11671-010-9603-4
[162] Hariharan R., Senthilkumar S., Suganthi A., Rajarajan M., (2012), Synthesis and characterization of doxorubicin modified ZnO/PEG nanomaterials and its photodynamic action. J. Photochem. and Photobiol. B: Biol. 116: 56-65. https://doi.org/https://doi.org/10.1016/j.jphotobiol.2012.08.008
[163] Brezaniova I., Hruby M., Kralova J., Kral V., Cernochova Z., Cernoch P., Slouf M., Kredatusova J., Stepanek P., (2016), Temoporfin-loaded 1-tetradecanol-based thermoresponsive solid lipid nanoparticles for photodynamic therapy. J. Cont. Releas. 241: 34-44. https://doi.org/https://doi.org/10.1016/j.jconrel.2016.09.009
[164] Wu J., Lin Y., Li H., Jin Q., Ji J., (2017), Zwitterionic stealth peptide-capped 5-aminolevulinic acid prodrug nanoparticles for targeted photodynamic therapy. J. Colloid Interf. Sci. 485: 251-259. https://doi.org/https://doi.org/10.1016/j.jcis.2016.09.012
[165] Kavelin V., Fesenko O., Dubyna H., Vidal C., Klar T. A., Hrelescu C., Dolgov L., (2017), Raman and luminescent spectra of sulfonated Zn phthalocyanine enhanced by gold nanoparticles. Nanosc. Res. Lett. 12: 197-205. https://doi.org/10.1186/s11671-017-1972-5
[166] Goto P. L., Siqueira-Moura M. P., Tedesco A. C., (2017), Application of aluminum chloride phthalocyanine-loaded solid lipid nanoparticles for photodynamic inactivation of melanoma cells. Int. J. Pharmac. 518: 228-241. https://doi.org/https://doi.org/10.1016/j.ijpharm.2017.01.004
[167] Lamch Ł., Bazylińska U., Kulbacka J., Pietkiewicz J., Bieżuńska-Kusiak K., Wilk K. A., (2014), Polymeric micelles for enhanced Photofrin II® delivery, cytotoxicity and pro-apoptotic activity in human breast and ovarian cancer cells. Photodiag. Photodyn. Therap. 11: 570-585. https://doi.org/https://doi.org/10.1016/j.pdpdt.2014.10.005
[168] da Volta Soares M., Oliveira M. R., dos Santos E. P., de Brito Gitirana L., Barbosa G. M., Quaresma C. H., Ricci-Júnior E., (2011), Nanostructured delivery system for zinc phthalocyanine: Preparation, characterization, and phototoxicity study against human lung adenocarcinoma A549 cells. Int. J. Nanomedic. 6: 227-238. https://doi.org/10.2147/IJN.S15860
[169] Natesan S., Krishnaswami V., Ponnusamy C., Madiyalakan M., Woo T., Palanisamy R., (2017), Hypocrellin B and nano silver loaded polymeric nanoparticles: Enhanced generation of singlet oxygen for improved photodynamic therapy. Mater. Sci. Eng: C. 77: 935-946. https://doi.org/https://doi.org/10.1016/j.msec.2017.03.179
[170] Fakayode O. J., Kruger C. A., Songca S. P., Abrahamse H., Oluwafemi O. S., (2018), Photodynamic therapy evaluation of methoxypolyethyleneglycol-thiol-SPIONs-gold-meso-tetrakis(4-hydroxyphenyl)porphyrin conjugate against breast cancer cells. Mater. Sci. Eng: C. 92: 737-744. https://doi.org/https://doi.org/10.1016/j.msec.2018.07.026
[171] Wang S., Fan W., Kim G., Hah H. J., Lee Y.-E. K., Kopelman R., Ethirajan M., Gupta, A., Goswami L. N., Pera P., Morgan J., Pandey R. K., (2011), Novel methods to incorporate photosensitizers into nanocarriers for cancer treatment by photodynamic therapy. Lasers in Surgery and Medic. 43: 686-695. https://doi.org/https://doi.org/10.1002/lsm.21113
[172] Ashkbar A., Rezaei F., Attari F., Ashkevarian S., (2020), Treatment of breast cancer in vivo by dual photodynamic and photothermal approaches with the aid of curcumin photosensitizer and magnetic nanoparticles. Sci. Rep. 10: 21206-21211. https://doi.org/10.1038/s41598-020-78241-1
[173] Zhao Z., Shi S., Huang Y., Tang S., Chen X., (2014), Simultaneous photodynamic and photothermal therapy using photosensitizer-functionalized Pd nanosheets by single continuous wave laser. ACS Appl. Mater. Interf. 6: 8878-8885. https://doi.org/10.1021/am501608c
[174] Qian H. S., Guo H. C., Ho P. C.-L., Mahendran R., Zhang Y., (2009), Mesoporous-silica-coated up-conversion fluorescent nanoparticles for photodynamic therapy. Small. 5: 2285-2290. https://doi.org/https://doi.org/10.1002/smll.200900692
[175] Idris N. M., Gnanasammandhan M. K., Zhang J., Ho P. C., Mahendran R., Zhang Y., (2012), In vivo photodynamic therapy using upconversion nanoparticles as remote-controlled nanotransducers. Nature Medic. 18: 1580-1585. https://doi.org/10.1038/nm.2933
[176] Yang X., Xiao Q., Niu C., Jin N., Ouyang J., Xiao X., He D., (2013), Multifunctional core–shell upconversion nanoparticles for targeted tumor cells induced by near-infrared light [10.1039/C3TB00575E]. J. Mater. Chem. B. 1: 2757-2763. https://doi.org/10.1039/C3TB00575E
[177] Ai F., Wang N., Zhang X., Sun T., Zhu Q., Kong W., Wang F., Zhu G., (2018), An upconversion nanoplatform with extracellular pH-driven tumor-targeting ability for improved photodynamic therapy [10.1039/C7NR06874C]. Nanoscale. 10: 4432-4441. https://doi.org/10.1039/C7NR06874C