Preparation, the physicochemical assessment, and the cytotoxicity of Cisplatin-loaded mesoporous Silica nanoparticles against head and neck squamous cell carcinoma cell line
Authors
- Simin Sharifi 1
- Elaheh Dalir Abdolahinia 2
-
Solmaz Maleki Dizaj
*
 3
3
- Touraj Nejatian 4
- Zahra Seydi 3
- Maryam Kouhsoltani 5
-
Yashar Rezaei
 3
3
- Masumeh Mokhtarpour 6
Abstract
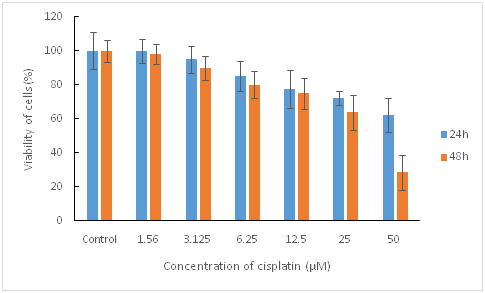
The aim of this study was to prepare, the physicochemical assessment and the cytotoxicity testing of cisplatin-loaded mesoporous silica nanoparticles against head and neck squamous cell carcinoma cell line (HNSCC). Cisplatin-loaded mesoporous silica nanoparticles were prepared through the precipitation method. The prepared nanoparticles were evaluated by conventional methods in terms of physicochemical properties. The cytotoxic effect of the nanoparticles and the free cisplatin were assessed on the head and neck squamous carcinoma cell line. The results showed that the prepared nanoparticles with nanometer size and the negative surface charge belonged to the MCM-41 silica family. TEM images established the mesoporous construction and the rod-shaped morphology of the produced nanoparticles. Based on Brunauer-Emmett-Teller (BET) analysis, the specific surface area, pore volume, and pore diameter decreased compared to free mesoporous silica because of drug filling into the mesoporous pores. The nanoparticles showed a two stage release pattern that continued slowly until the 35th day. Mesoporous silica nanoparticles displayed no significant cytotoxic effect on HNSCC. Cisplatin displayed a cytotoxic effect with IC50 of 82.01 μM and 33.67 μM in 24 h and 48 h incubation times, respectively. However, cisplatin-loaded mesoporous silica nanoparticles displayed a cytotoxic effect with IC50 of 26.17 μM and 13.28 μM in 24 and 48 h incubation times, respectively. The results can highlight the capability of cisplatin-loaded mesoporous silica nanoparticles to be applied in the treatment of oral cancerous cells.
Graphical Abstract
Keywords
References
1. Zheng W., Zhou Q., Yuan C. J. B. C., (2021), Nanoparticles for oral cancer diagnosis and therapy. Bioinorg. Chem. Appl. 2021: Article ID 9977131.
2. Balogh J., Victor III D., Asham E. H., Burroughs S. G., Boktour M., Saharia A., (2016), Hepatocellular carcinoma: A review. J. Hepatocell Carcinoma. 3: 41-53.
3. Kirtane A. R., Verma M., Karandikar P., Furin J., Langer R., Traverso G. J. N. N., (2021), Nanotechnology approaches for global infectious diseases. Nat. Nanotechnol. 16: 369-384.
4. Poole Jr. C. P., Owens F. J., (2003), Introduction to Nanotechnology. John Wiley & Sons.
5. McNeil S. E. J. J., (2005), Nanotechnology for the biologist. Leukoc Biol. 78: 585-594.
6. Su Z., Dong S., Zhao S.-C., Liu K., Tan Y., Jiang X., (2021), Novel nanomedicines to overcome cancer multidrug resistance. Drug Resist. Updat. 58: 100777-100785.
7. Agnello L., Tortorella S., d’Argenio A., Carbone C., Camorani S., Locatelli E., (2021), Optimizing cisplatin delivery to triple-negative breast cancer through novel EGFR aptamer-conjugated polymeric nanovectors. J. Exp. Clinic. Cancer Res. 40: Article number: 239.
8. Duan X., He C., Kron S. J., Lin W., (2016), Nanoparticle formulations of cisplatin for cancer therapy. Wiley Interdisc. Rev: Nanomedic. Nanobiotechnol. 8: 776-791.
9. George M., Pejovic M., Thuaire M., Kramar A., Wolff J. J. B., (1983), Randomized comparative trial of a new anti-emetic: Nabilone, in cancer patients treated with cisplatin. Biomed. Pharmacother. 37: 24-27.
10. Nakatomi C., Hitomi S., Yamaguchi K., Hsu C.-C., Seta Y., Harano N., (2022), Cisplatin induces TRPA1-mediated mechanical allodynia in the oral mucosa. Arch. Oral Biol. 133: 105317-105322.
11. Ahmadi R., Jalali Sarvestani M. R., Sadeghi B., (2018), Computational study of the fullerene effects on the properties of 16 different drugs: A review. Int. J. Nano Dimens. 9: 325-335.
12. Athinarayanan J., Periasamy V. S., Alhazmi M., Alatiah K. A., Alshatwi A. A., (2015), Synthesis of biogenic silica nanoparticles from rice husks for biomedical applications. Cerm. Int. 41: 275-281.
13. Ghaferi M., Koohi Moftakhari Esfahani M., Raza A., Al Harthi S., Ebrahimi Shahmabadi H., Alavi S. E., (2021), Mesoporous silica nanoparticles: Synthesis methods and their therapeutic use-recent advances. Drug Target. 29: 131-154.
14. Vahdati A. R., Sadeghi B., (2013), A study on the assessment of DNA strand-breaking activity by silver and silica nanoparticles. J. Nanostruc. Chem. 3: Article number: 7.
15. Memar M. Y., Yekani M., Ghanbari H., Shahi S., Sharifi S., Maleki Dizaj S., (2020), Biocompatibility, cytotoxicity and antibacterial effects of meropenem-loaded mesoporous silica nanoparticles against carbapenem-resistant Enterobacteriaceae. Artif. Cells Nanomed. Biotechnol. 48: 1354-1361.
16. Almukainzi M., Okumu A., Wei H., Löbenberg R., (2015), Simulation of in vitro dissolution behavior using DDDPlus™. AAPS. Pharm. Sci. Tech. 16: 217-221.
17. Chen J., Zhang S., Zhang S., Gao S., Wang J., Lei D., (2019), Mesoporous silica nanoparticle–based combination of NQO1 inhibitor and 5-Fluorouracil for potent antitumor effect against head and neck squamous cell carcinoma (HNSCC). Nanoscale Res. Lett.14: 1-12.
18. Dowding J. M., Das S., Kumar A., Dosani T., Mc Cormack R., Gupta A., (2013), Cellular interaction and toxicity depend on physicochemical properties and surface modification of redox-active nanomaterials. ACS Nano. 7: 4855-4868.
19. Bakand S., Hayes A., (2016), Toxicological considerations, toxicity assessment, and risk management of inhaled nanoparticles. Int. J. Mol. Sci. 17: 929-935.
20. Mitchell M. J., Billingsley M. M., Haley R. M., Wechsler M. E., Peppas N. A., Langer R., (2021), Engineering precision nanoparticles for drug delivery. Nat. Rev. Drug Discov. 20: 101-124.
21. Lowry G. V., Hill R. J., Harper S., Rawle A. F., Hendren C. O., Klaessig F., (2016), Guidance to improve the scientific value of zeta-potential measurements in nano EHS. Environ. Sci: Nano. 3: 953-965.
22. Naushad M., Alqadami A. A., AlOthman Z. A., Alsohaimi I. H., Algamdi M. S., Aldawsari A. M., (2019), Adsorption kinetics, isotherm and reusability studies for the removal of cationic dye from aqueous medium using arginine modified activated carbon. J. Mol. Liq. 293: 111442-111448.
23. Tavakoli P., Shadizadeh S. R., Hayati F., Fattahi M., (2020), Effects of synthesized nanoparticles and Henna-Tragacanth solutions on oil/water interfacial tension: Nanofluids stability considerations. Petroleom. 6: 293-303.
24. Geng Y., Dalhaimer P., Cai S., Tsai R., Tewari M., Minko T., (2007), Shape effects of filaments versus spherical particles in flow and drug delivery. Nat. Nanotech. 2: 249-255.
25. Li D., Tang Z., Gao Y., Sun H., Zhou Sh., (2016), A bioinspired rodshaped nanoplatform for strongly infecting tumor cells and enhancing the delivery efficiency of anticancer drugs. Adv. Funct. Mater. 26: 66-79.
26. Zhao Y., Wang Y., Ran F., Cui Y., Liu C., Zhao Q., Gao Y., Wang D., Wang S., (2017), A comparison between sphere and rod nanoparticles regarding their in vivo biological behavior and pharmacokinetics. Science Rep. 7: Article number: 4131.
27. Costa J. A. S., de Jesus R. A., Santos D. O., Mano J. F., Romao L. P., Paranhos C. M., (2020), Recent progresses in the adsorption of organic, inorganic, and gas compounds by MCM-41-based mesoporous materials. Microp. Mesopr. Mater. 291: 109698-109719.
28. Rotureau P., (2001), Study of water radiolysis in porous media.Service Chemia Molcula.URA 331, IAEA.
29. Karimzadeh M., Rashidi L., Ganji F., (2017), Mesoporous silica nanoparticles for efficient rivastigmine hydrogen tartrate delivery into SY5Y cells. Drug. Dev. Ind. Pharm. 43: 628-636.
30. Pedersen B. T., Larsen S. W., Østergaard J., Larsen C., (2008), In vitro assessment of lidocaine release from aqueous and oil solutions and from preformed and in situ formed aqueous and oil suspensions. Parenteral depots for intra-articular administration. Drug Deliv.15: 23-30.
31. Bhattacharyya S., Wang H., Ducheyne P., (2012), Polymer-coated mesoporous silica nanoparticles for the controlled release of macromolecules. Acta Biomater. 8: 3429-3435.
32. Memar M. Y., Yekani M., Ghanbari H., Nabizadeh E., Vahed S. Z., Dizaj S. M., (2021), Antimicrobial and antibiofilm activities of meropenem loaded-mesoporous silica nanoparticles against carbapenem-resistant Pseudomonas aeruginosa. J. Biomater. Appl. 36: 605-612.
33. Yuan Z., Pan Y., Cheng R., Sheng L., Wu W., Pan G., (2016), Doxorubicin-loaded mesoporous silica nanoparticle composite nanofibers for long-term adjustments of tumor apoptosis. Nanotechnol. 27: 245101-245106.
34. Senior B. A., Kennedy D. W., (1996), Management of sinusitis in the asthmatic patient. Annals of Allergy, Asthma & Immunol. 77: 6-19.
35. Karimzadeh M., Rashidi L., Ganji F., (2017), Mesoporous silica nanoparticles for efficient rivastigmine hydrogen tartrate delivery into SY5Y cells. Drug Develop. Indus. Pharm. 43: 628-636.
36. Sato I., Umemura M., Mitsudo K., Kioi M., Nakashima H., Iwai T., (2014), Hyperthermia generated with ferucarbotran (Resovist®) in an alternating magnetic field enhances cisplatin-induced apoptosis of cultured human oral cancer cells. The J. Physilogic. Sci. 64: 177-183.
37. Cooley M. E., Davis L., Abrahm J., (1994), Cisplatin: A clinical review. Part II--Nursing assessment and management of side effects of cisplatin. Cancer Nurs. 17: 283-293.
38. Li W., Zhang H., Assaraf Y. G., Zhao K., Xu X., Xie J., (2016), Overcoming ABC transporter-mediated multidrug resistance: Molecular mechanisms and novel therapeutic drug strategies. Drug. Resis. Update. 27: 14-29.
39. Hu C.-M., Zhang L., (2012), Nanoparticle-based combination therapy toward overcoming drug resistance in cancer. Biochem Pharmacol. 83: 1104-1111.
40. Wang D., Yu D., Liu X., Wang Q., Chen X., Hu X., Wang Q., Jin Ch., Wen L., Zhang L., (2020), Targeting laryngeal cancer cells with 5-fluorouracil and curcumin using mesoporous silica nanoparticles. Technol. Cancer Res. Treat. 19: 1533033820962114.
41. Martínez-Edo G., Fornaguera C., Borrós S., and Sánchez-García D.J.P., (2020), Glycyrrhetinic acid-functionalized mesoporous silica nanoparticles for the co-delivery of DOX/CPT-PEG for targeting HepG2 Cells. Pharmaceu. 12: 1048-1055.