Photocatalytic degradation studies of malachite green dye by hydrothermally synthesized Cobalt Vanadate nanoparticles
Authors
Abstract
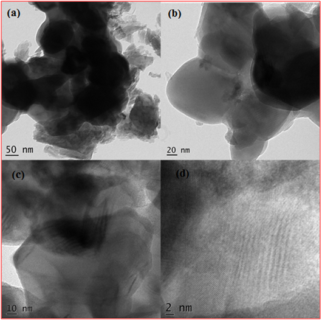
Cobalt vanadate (Co3V2O8) nanoparticles were prepared by a hydrothermal process using ammonia metavanadate and cobalt nitrate as precursors and calcined at 450 °C to obtain pure Co3V2O8 nanoparticles. The sample was characterized by scanning electron microscopy, X-ray diffraction, Fourier transform infrared, and UV-visible diffuse reflectance spectroscopy. The characterization analysis confirmed variations in the structure, shape, functional group, and energy gap of Co3V2O8 nanoparticles. Using the Tauc relationship, the energy band gap was determined by analyzing the Tauc curve. The nanoparticles obtained had an average size of 40 nm and found the zeta potential of the nanoparticles was -78 mV, indicating good dispersion and stability. The photocatalytic activity of Co3V2O8 nanoparticles through the degradation of malachite green dye was investigated under UV light irradiation. According to the results, Co3V2O8 nanoparticles showed a maximum removal efficiency of 89 percent in 60 minutes. It shows that synthesized Co3V2O8 nanoparticles have a strong potential for application as a photocatalyst to degrade textile dyes in wastewater treatment rapidly.
Graphical Abstract
Keywords
- Degradation
- hydrothermal synthesis
- Cobalt Vanadate
- Wastewater treatment
- Photocatalyst
- Malachite green
References
1. Kanwar V. S., Sharma A., Srivastav A. L., Rani L., (2020), Phytoremediation of toxic metals present in soil and water environment: A critical review. Sci. Pollut. Res.27: 44835-44860.
2. Verma M., Mitan M., Kim H., Vaya D., (2021), Efficient photocatalytic degradation of Malachite green dye using facilely synthesized cobalt oxide nanomaterials using citric acid and oleic acid. Phys. Chem. Solids.155: 110125-110129.
3. Lima D. R., Klein, L., Dotto, G. L., (2017), Application of ultrasound modified corn straw as adsorbent for malachite green removal from synthetic and real effluents. Sci. Pollut. Res. 24: 21484-21495.
4. Naik R. L., Narsaiah T. B., (2022), Hydrothermal synthesis and characterization of nanocrystalline zinc vanadate (Zn2V2O7) on graphene oxide scaffolds. Materials Today: Proceed. In Press.
5. Oplatowska-Stachowiak M., Elliott C. T., (2017), Food colors: Existing and emerging food safety concerns. Rev. Food Sci. Nutrit. 57: 524-548.
6. Sang D. K., Wang H., Guo Z., Xie N., Zhang H., (2019), Recent developments in stability and passivation techniques of phosphorene toward next‐generation device applications. Func. Mater. 29: 1903419.
7. Tong H., Ouyang S., Bi Y., Umezawa N., Oshikiri M., Ye J., (2012), Nano‐photocatalytic materials: Possibilities and challenges. Mater. 24: 229-251.
8. Naciri Y., Hsini A., Ajmal Z., Navío J. A., Bakiz B., Albourine A., Benlhachemi A., (2020), Recent progress on the enhancement of photocatalytic properties of BiPO4 using π–conjugated materials. Colloid and Interf. Sci.280: 102160.
9. Chen X., Mao S. S., (2007), Titanium dioxide nanomaterials: Synthesis, properties, modifications, and applications. Rev.107: 2891-2959.
10. Maduraiveeran G., Sasidharan M., Jin W., (2019), Earth-abundant transition metal and metal oxide nanomaterials: Synthesis and electrochemical applications. Mater. Sci.106: 100574.
11. Kayani A. B. A., Kuriakose S., Monshipouri M., Khalid F. A., Walia S., Sriram S., Bhaskaran M., (2021), UV photochromism in transition metal oxides and hybrid materials. Small. 17: 2100621.
12. Kalantar-zadeh K., Ou J. Z., Daeneke T., Mitchell A., Sasaki T., Fuhrer M. S., (2016), Two dimensional and layered transition metal oxides. Mater. Today. 5: 73-89.
13. Azadmanjiri J., Kumar P., Srivastava V. K., Sofer Z., (2020), Surface functionalization of 2D transition metal oxides and dichalcogenides via covalent and non-covalent bonding for sustainable energy and biomedical applications. ACS Appl. Nano Mater.3: 3116-3143.
14. Monga D., Sharma S., Shetti N. P., Basu S., Reddy K. R., Aminabhavi T. M., (2021), Advances in transition metal dichalcogenide-based two-dimensional nanomaterials. Today Chem. 19: 100399.
15. Naik R. L., Justin P., Narsaiah T. B., (2020), Controllable synthesis of cobalt vanadate nanostructure materials for direct methanol fuel cell applications. In Proceed. Int. Conf. Adv. Chem. Eng.(AdChE).
16. Ramavathu L. N., Harapanahalli S. R., Pernapati N., Tumma B. N., (2021), Synthesis and characterization of Nickel Metavanadate (Ni3V2O8)-application as photocatalyst and supercapacitor. J. Nano Dimens. 12: 411-421.
17. Keerthana S. P., Yuvakkumar R., Kumar P. S., Ravi G., Velauthapillai D., (2022), Surfactant induced copper vanadate (β-Cu2V2O7, Cu3V2O8) for different textile dyes degradation. Res. 112964.
18. Ramavathu L. N., Maniam K. K., Gopalram K., Chetty R., (2012), Effect of pyrolysis temperature on cobalt phthalocyanine supported on carbon nanotubes for oxygen reduction reaction. Appl. Electrochem. 42: 945-951.
19. Lakshmana Naik R., Bala Narsaiah T., Ravikumar C. R., Naveen Kumar A., Vinutha K., Jahagirdar A. A., Ananda Murthy H. C., (2021), Synthesis and characterization of nickel cobalt vanadate (NiCo2V2O8) nanostructures: Photocatalytic and supercapacitor applications. Asian J. Chem. 33: 2831-2838.
20. Lakshmana Naik R., Justin P., Narsaiah B., (2020), Size controlled hydrothermal synthesis and characterization of Nickel Metavanadate (NiVO3) nanoparticles. J. Adv. Sci. Technol. 29: 10012 – 10018.
21. Naveen Kumar Reddy K., Lakshmana Naik R., Narsaiah Tumma B., (2021), Modelling and simulation of advanced Alkaline Water electrolysis. Adv. Res. J. Sci. Eng. Technol. 8: 729-734.
22. Bouchareb R., Bilici Z., Dizge N., (2021), Potato processing wastewater treatment using a combined process of chemical coagulation and membrane filtration. Clean Soil, Air, Water.49: 2100017.
23. Zhao C., Zhou J., Yan Y., Yang L., Xing G., Li H., Zheng H., (2021), Application of coagulation/flocculation in oily wastewater treatment: A review. TotalEenvironm. 765: 142795.
24. Gisbertz S., Pieber B., (2020), Heterogeneous photocatalysis in organic synthesis. Photo. Chem. 4: 456-475.
25. Lervolino G., Zammit I., Vaiano V., Rizzo L., (2019), Limitations and prospects for wastewater treatment by UV and visible-light-active heterogeneous photocatalysis: A critical review.Top Current Chem. 387: 31840195.
26. Ray P. C., (2010), Size and shape dependent second order nonlinear optical properties of nanomaterials and their application in biological and chemical sensing. Rev. 110: 5332-5365.
27. Hashim A., Hadi Q., (2018), Structural, electrical and optical properties of (biopolymer blend/titanium carbide) nanocomposites for low cost humidity sensors. Mater. Sci: Mater. Electron.29: 11598-11604.
28. Pokhrel S., Mädler L., (2020), Flame-made particles for sensors, catalysis, and energy storage applications.Energy Fuels.34: 13209-13224.
29. Budnyak T. M., Slabon A., Sipponen M. H., (2020), Lignin–inorganic interfaces: Chemistry and applications from adsorbents to catalysts and energy storage materials. Sus. Chem. 13: 4344-4355.
30. Hakimyfard A., Khademinia S., (2022), Hirshfeld surface analysis of solid-state synthesized NiFe2O4 nanocomposite and application of it for photocatalytic degradation of Water pollutant dye. J. Nano Dimens. 13: 155-167.
31. Nandi D., Mohan V. B., Bhowmick A. K., Bhattacharyya D., (2020), Metal/metal oxide decorated graphene synthesis and application as supercapacitor: A review. Mater. Sci.55: 6375-6400.
32. Tareen A. K., Priyanga G. S., Behara S., Thomas T., Yang M., (2019), Mixed ternary transition metal nitrides: A comprehensive review of synthesis, electronic structure, and properties of engineering relevance. Solid State Chem. 53: 1-26.
33. Verma M., Mitan M., Kim H., Vaya D., (2021), Efficient photocatalytic degradation of Malachite green dye using facilely synthesized cobalt oxide nanomaterials using citric acid and oleic acid. Phys. Chem. Solids.155: 110125.
34. Yang J., Wu M., Gong F., Feng T., Chen C., Liao J., (2017), Facile and controllable synthesis of solid Co3V2O8 micro-pencils as a highly efficient anode for Li-ion batteries. RSC Adv. 7: 24418-24424.
35. Amulya M. S., Nagaswarupa H. P., Kumar M. A., Ravikumar C. R., Prashantha S. C., Kusuma K. B., (2020), Sonochemical synthesis of NiFe2O4 nanoparticles: Characterization and their photocatalytic and electrochemical applications. Surf. Sci. Adv. 1: 100023.
36. O’Dwyer C., Gannon G., McNulty D., Buckley D. N., Thompson D., (2012), Accommodating curvature in a highly ordered functionalized metal oxide nanofiber: Synthesis, characterization, and multiscale modeling of layered nanosheets. Chem Mater. 24: 3981-3992.
37. Aydoghmish S. M., Hassanzadeh-Tabrizi S. A., Saffar-Teluri A., (2019), Facile synthesis and investigation of NiO–ZnO–Ag nanocomposites as efficient photocatalysts for degradation of methylene blue dye. Int. 45: 14934-14942.
38. Xiao M., Yang D., Yan Y., Tian Y., Zhou M., Hao M., Miao Y., (2015), Nanoplates and nanospheres of Co3 (VO4)2 as noble metal-free electrocatalysts for oxygen evolution. Acta. 180: 260-267.
39. Xing M., Kong L. B., Liu M. C., Liu L. Y., Kang L., Luo Y. C., (2014), Cobalt vanadate as highly active, stable, noble metal-free oxygen evolution electrocatalyst. Mater. Chem. A. 2: 18435-18443.
40. Hu L., Shang C., (2020), Co3V2O8 nanoparticles supported on reduced graphene oxide for efficient lithium storage. 10: 740-746.
41. Youn C., Shin S., Shin K., Kim C., Park C. L., Choi J., Kim S. H., Yeo S. Y., Shin M. W., Henkelman G., Yoon K. R., (2022), Template-assisted synthesis of single-atom catalysts supported on highly crystalline vanadium pentoxide for stable oxygen evolution. Catalysis. 2: 1-20.
42. Ijaz F., Shahid S., Khan S. A., Ahmad W., Zaman S., (2017), Green synthesis of copper oxide nanoparticles using a butilon indicum leaf extract: Antimicrobial, antioxidant and photocatalytic dye degradation activitie.Tropical J. Pharmac. Res. 16: 743-753.
43. Mafa P. J., Malefane M. E., Idris A. O., Mamba B. B., Liu D., Gui J., Kuvarega A. T., (2021), Cobalt oxide/copper bismuth oxide/samarium vanadate (Co3O4/CuBi2O4/SmVO4) dual Z-scheme heterostructures photocatalyst with high charge-transfer efficiency: Enhanced carbamazepine degradation under visible light irradiation. Colloid Interf. Sci. 603: 666-684.
44. Faniband M., Vidyasagar C. C., Jimenez V., Shridhar A. H., (2022), Mechanistic insight into the photocatalytic degradation of organic pollutant and electrochemical behavior of modified MWCNTs/Cu-Co3O4 nanocomposite. React. Chem. Eng. 7: 1847-1872.
45. Parsa M. M., Pourfakhar H., Baghdadi M., (2020), Application of graphene oxide nanosheets in the coagulation-flocculation process for removal of Total Organic Carbon (TOC) from surface water. J. Water Proc. Eng. 37: 101367.
46. Azari B., Pourahmad A., Sadeghi B., Mokhtary M., (2019), Preparation and photocatalytic study of SiO2/CuS coreshell nanomaterial for degradation of methylene blue dye. Nanoscale. 6: 103-114.
47. Zhao Y., Liu Y., Du X., Han R., Ding Y., (2014), Hexagonal assembly of Co3V2O8 nanoparticles acting as an efficient catalyst for visible light-driven water oxidation. Mater. Chem. A. 2: 19308-19314.
48. Zhang H., Guan W., Zhang L., Guan X., Wang S., (2020), Degradation of an organic dye by bisulfite catalytically activated with iron manganese oxides: The role of superoxide radicals. ACS Omega. 5: 18007-18012.
49. Alsulami Q. A., Rajeh A., Mannaa M. A., Albukhari S. M., Baamer D. F., (2021), Preparation of highly efficient sunlight driven photodegradation of some organic pollutants and H2 evolution over rGO/FeVO4 Int. J. Hydrogen Energy. 46: 27349–27363.
50. Arsalani N., Bazazi S., Abuali M., Jodeyri S., (2019), A new method for preparing ZnO/CNT nanocomposites with enhanced photocatalytic degradation of malachite green under visible light. Photochem. Photobiol. A: Chem. 389: 112207.
51. Verma M., Mitan M., Kim H., Vaya D., (2021), Efficient photocatalytic degradation of Malachite green dye using facilely synthesized cobalt oxide nanomaterials using citric acid and oleic acid. Phys. Chem. Solids. 155: 110125.
52. Zhang M., Gong J., Zeng G., Zhang P., Song B., Cao W., Huan S., (2018), Enhanced degradation performance of organic dyes removal by bismuth vanadate-reduced graphene oxide composites under visible light radiation. Colloids Surf. A: Physicochem. Eng. Asp. 559: 169-183.