Comparative study on Pb2+ adsorption using spinel nanostructures-embedded Graphene Oxide
Authors
Abstract
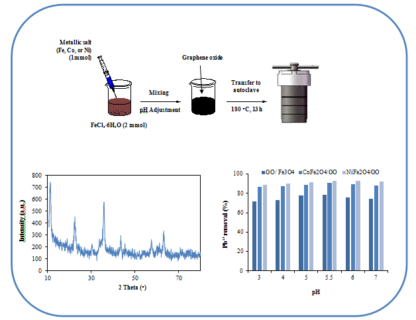
This study investigates the adsorption performance of spinel nanostructures-embedded graphene oxide (GO) for removing toxic Pb2+ ions from water. The graphene oxide nanocomposites containing Fe3O4, CoFe2O4, and NiFe2O4 nanoparticles were synthesized and characterized. The NiFe2O4/GO nanocomposite exhibited the highest Pb2+ adsorption capacity, reaching 96.1% removal at 75 minutes contact time with initial Pb2+ concentration of 10 ppm. The CoFe2O4/GO nanocomposite achieved 90.3% Pb2+ removal under similar conditions, while Fe3O4/GO showed 88.8%. Kinetic studies revealed the adsorption followed a pseudo-second-order model with R2 values of 0.99-1.00. The adsorption mechanism involves surface adsorption on GO sheets and spinel nanoparticles. The nanocomposites demonstrated good recyclability, maintaining over 65% removal efficiency after three consecutive cycles. This study provides insights into designing effective adsorbents for mitigating potentially toxic elements pollution in water, addressing environmental and public health challenges.
Graphical Abstract
Read the full text of the articleKeywords
References
[1] Swain C. K., (2024), Environmental pollution indices: A review on concentration of heavy metals in air, water, and soil near industrialization and urbanization. Discover Environ. 2: 5-9. https://doi.org/10.1007/s44274-024-00030-8
[2] Das S., Sultana K. W., Ndhlala A. R., Mondal M., Chandra I., (2023), Heavy Metal Pollution in the Environment and Its Impact on Health: Exploring Green Technology for Remediation. Environ. Health Insights. 17: 1-10. https://doi.org/10.1177/11786302231201259
[3] Wani A. L., Ara A., Usmani J. A., (2015), Lead toxicity: A review. Interdiscip. Toxicol. 8: 55-64. https://doi.org/10.1515/intox-2015-0009
[4] Raji Z., Karim A., Karam A., Khalloufi S., (2023), Adsorption of heavy metals: mechanisms, kinetics, and applications of various adsorbents in wastewater remediation-a review. Waste. 1: 775-805. https://doi.org/10.3390/waste1030046
[5] Hosseini N.S., Sobhanardakani S., Cheraghi M., Lorestani B., Merrikhpour H., (2020), Heavy metal concentrations in roadside plants (Achillea wilhelmsii and Cardaria draba) and soils along some highways in Hamedan, west of Iran. Environ. Sci. Pollut. Res. 27:13301-13314. https://doi.org/10.1007/s11356-020-07874-6
[6] Sobhanardakani S., Ahmadi M., Zandipak R., (2016), Efficient removal of Cu(II) and Pb(II) heavy metal ions from water samples using 2, 4-dinitrophenylhydrazine loaded sodium dodecyl sulfate-coated magnetite nanoparticles. J. Water Supply: Res. Technol. – AQUA 65: 361-372. https://doi.org/10.2166/aqua.2016.100
[7] Omidi A.H., Cheraghi M., Lorestani B., Sobhanardakani S., Jafari A., (2019), Biochar obtained from cinnamon and cannabis as effective adsorbents for removal of lead ions from water. Environ. Sci. Pollut. Res. 26: 27905-27914. https://doi.org/10.1007/s11356-019-05997-z
[8] Soylak M., Ozalp O, Uzcan F., (2021), Magnetic nanomaterials for the removal, separation and preconcentration of organic and inorganic pollutants at trace levels and their practical applications: A review. Trends Environ. Anal. Chem. 29: e00109. http://dx.doi.org/10.1016/j.teac.2020.e00109
[9] Soylak M., Alasaad M., Özalp Ö., (2022) Fabrication and characterization of MgCo2O4 for solid phase extraction of Pb(II) from environmental samples and its detection with high-resolution continuum source flame atomic absorption spectrometry (HR-CS-FAAS). Microchem. J. 178: 107329. https://doi.org/10.1016/j.microc.2022.107329
[10] Uzcan F., Soylak M., (2021), CuCo2O4 as affective adsorbent for dispersive solid‑phase extraction of lead from food, cigarette and water samples before FAAS detection. Chem. Pap. 75: 6367-6375. https://doi.org/10.1007/s11696-021-01797-3
[11] Sen T. K., (2023), Agricultural solid wastes based adsorbent materials in the remediation of heavy metal ions from water and wastewater by adsorption: A review. Molecules. 28: 5575-5579. https://doi.org/10.3390/molecules28145575
[12] Gisi S. D., Lofrano G., Grassi M., Notarnicola M., (2016), Characteristics and adsorption capacities of low-cost sorbents for wastewater treatment: A review. Sustain. Mater. Technol. 9: 10-40. http://dx.doi.org/10.1016/j.susmat.2016.06.002
[13] Iqbal Z., Tanweer M. S., Masood Alam M., (2023), Reduced graphene oxide-modified spinel cobalt ferrite nanocomposite: synthesis, characterization, and its superior adsorption performance for dyes and heavy metals. ACS Omega 8: 6376-6390. https://doi.org/10.1021/acsomega.2c06636
[14] Narayana P. L., Lingamdinne L. P., Karri R. R., Devanesan S., AlSalhi M. S., Reddy N. S., Chang Y. Y., Koduru J. R., (2022), Predictive capability evaluation and optimization of Pb(II) removal by reduced graphene oxide-based inverse spinel nickel ferrite nanocomposites. Environ. Res. 204: 112029. https://doi.org/10.1016/j.envres.2021.112029
[15] Kumar R., Bhattacharya S., Sharma P., (2021), Novel insights into adsorption of heavy metal ions using magnetic graphene composites. J. Environ. Chem. Eng. 9: 106212. https://doi.org/10.1016/j.jece.2021.106212
[16] Lingamdinne L. P., Koduru J. R., Choi Y. L., Chang Y. Y., Jae-Kyu Yang J. K., (2016), Studies on removal of Pb(II) and Cr(III) using graphene oxide based inverse spinel nickel ferrite nano-composite as sorbent. Hydrometallurgy. 165: 64-72. http://dx.doi.org/10.1016/j.hydromet.2015.11.005
[17] Parastar Gharehlar M., Sheshmani S., Nikmaram F. R., Doroudi Z., (2023), Assessing the efficacy of iron (II, III) oxide nanocomposites for the photodegradation of organic dye pollutants and textile wastewater under UV-visible irradiation. Chem. Pap. 3: 2015-2032. https://doi.org/10.1007/s11696-023-03223-2
[18] Parastar Gharehlar M., Sheshmani S., Nikmaram F. R., Doroudi Z., (2024), Synergistic potential in spinel ferrite MFe2O4 (M = Co, Ni) nanoparticles-mediated graphene oxide: Structural aspects, photocatalytic, and kinetic studies. Sci. Rep. 14: 4625-4629. https://doi.org/10.1038/s41598-024-55452-4
[19] Hosseinzadeh S., Eslami Moghadam M., Sheshmani S., Shahvelayati A.S., (2020), Some new anticancer platinum complexes of dithiocarbamate derivatives against human colorectal and pancreatic cell lines. J. Biomol. Struct. Dyn. 38: 2215-2228. https://doi.org/10.1080/07391102.2019.1627909
[20] Soleimannejad J., Aghabozorg H., Mohammadzadeh Y., Nasibipour M., Sheshmani S., Shokrollahi A., Karami E., Shamsipure M., (2011), Different complexation behavior of Fe(III), Co(II) and Ni(II) with pyridine-2,6-dicarboxylic Acid and 4,4′-bipyridine adduct: Syntheses, crystal structures and solution studies. J. Iran. Chem. Soc. 8: 247-264. https://doi.org/10.1007/BF03246222
[21] Aghabozorg H., Soleimannejad J., Sharif M., Sheshmani S., Moghimi A., (2005), Crystal structure of a proton-transfer compound between 2, 6-pyridinedicarboxylic acid and N,N′-diethyl-2-amino-6-methyl-4-pyrimidinol, Anal. SCi. X-ray Struc. Anal. 21: x73-x74. https://doi.org/10.2116/analscix.21.x73