Porphyrin-based metal–organic frameworks: focus on diagnostic and therapeutic applications
- Department of Chemistry, Faculty of Science, Ferdowsi University of Mashhad, Mashhad, IR
- Pharmaceutical Research Center, Pharmaceutical Technology Institute, Mashhad University of Medical Sciences, Mashhad, IR Department of Pharmaceutical Biotechnology, School of Pharmacy, Mashhad University of Medical Sciences, Mashhad, IR

Published in Issue 16-05-2022

This work is licensed under a Creative Commons Attribution 4.0 International License.
How to Cite
Hassanzadeh Goji, N., Ramezani, M., Sh. Saljooghi, A., & Alibolandi, M. (2022). Porphyrin-based metal–organic frameworks: focus on diagnostic and therapeutic applications. Journal of Nanostructure in Chemistry, 14(2 (April 2024). https://doi.org/10.1007/s40097-022-00500-6
Abstract
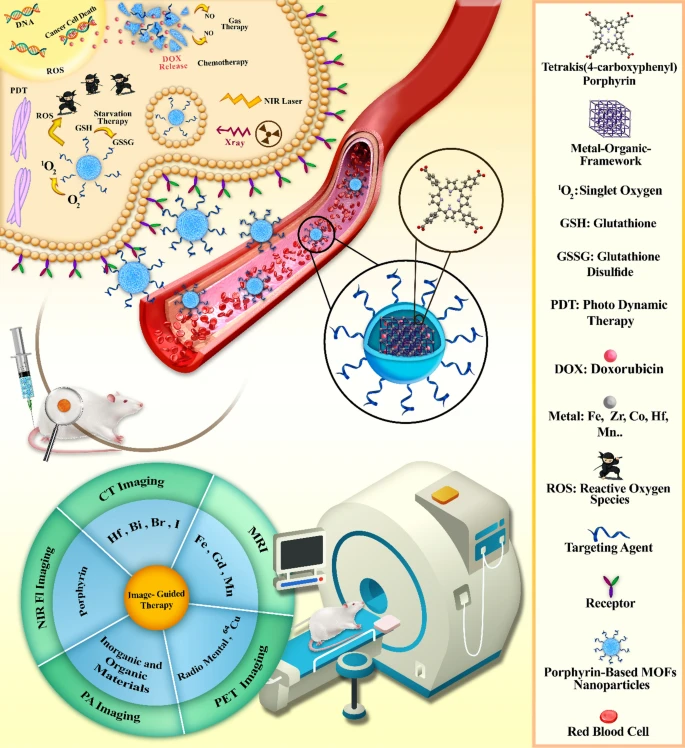
Abstract As a hybrid material, metal organic frameworks (MOFs) contain unique characteristics for biomedical applications such as high porosity, large surface area, different crystalline morphologies, and nanoscale dimensions. These frameworks are assembled through the interconnection of organic linkers with metal nodes, while the engineering of an MOF for biomedical applications requires versatile linkers with acceptable symmetry. Porphyrin, as an organic linker with interesting photochemical and photophysical properties, attracted the attention of many for engineering the potent multifunctional porphyrinic metal organic frameworks (PMOFs). In this regard, a large number of approaches were conducted for designing robust practical PMOFs with a wide range of applications. In this review, we introduced another perspective of MOFs and coordination polymers constructed from porphyrinic linkers with a special focus on those synthesized by meso-tetrakis (4-carboxyphenyl) porphyrin (TCPP). In the following, we summarized and discussed the different types of PMOFs and their biomedical applications in terms of diagnostic agent, therapeutic platform, and drug delivery vehicle. Graphical abstractKeywords
- Porphyrin,
- Metal organic frameworks,
- Coordination polymer,
- Drug delivery
References
- Xu et al. (2018) Two-dimensional metal–organic framework nanosheets: a rapidly growing class of versatile nanomaterials for gas separation, MALDI-TOF matrix and biomimetic applications 24(57) (pp. 15131-15142) https://doi.org/10.1002/chem.201800556
- Kuppler et al. (2009) Potential applications of metal–organic frameworks 253(23–24) (pp. 3042-3066) https://doi.org/10.1016/j.ccr.2009.05.019
- Zhou et al. (2012) Introduction to metal–organic frameworks 112(2) (pp. 673-674) https://doi.org/10.1021/cr300014x
- Kukkar et al. (2018) Recent progress in biological and chemical sensing by luminescent metal–organic frameworks (pp. 1346-1370) https://doi.org/10.1016/j.snb.2018.06.128
- Xie et al. (2011) A metalloporphyrin functionalized metal–organic framework for selective oxidization of styrene 47(19) (pp. 5521-5523)
- Gao et al. (2013) Two rare indium-based porous metal–metalloporphyrin frameworks exhibiting interesting CO2 uptake 15(45) (pp. 9320-9323) https://doi.org/10.1039/c3ce41090k
- Feng et al. (2013) Metal–organic frameworks based on previously unknown Zr8/Hf8 cubic clusters 52(21) (pp. 12661-12667) https://doi.org/10.1021/ic4018536
- Nandi and Goldberg (2014) Fixation of CO2 in bi-layered coordination networks of zinc tetra (4-carboxyphenyl) porphyrin with multi-component [Pr2Na3(NO3)(H2O)3] connectors 50(88) (pp. 13612-13615)
- Wang et al. (2014) A series of highly stable mesoporous metalloporphyrin Fe-MOFs 136(40) (pp. 13983-13986) https://doi.org/10.1021/ja507269n
- Beyzavi et al. (2015) A hafnium-based metal–organic framework as a nature-inspired tandem reaction catalyst 137(42) (pp. 13624-13631) https://doi.org/10.1021/jacs.5b08440
- Zhang et al. (2015) An efficient catalyst based on a metal metalloporphyrinic framework for highly selective oxidation 145(2) (pp. 589-595) https://doi.org/10.1007/s10562-014-1433-z
- Yuan et al. (2015) A single crystalline porphyrinic titanium metal–organic framework 6(7) (pp. 3926-3930) https://doi.org/10.1039/C5SC00916B
- Yamabayashi et al. (2018) Scaling up electronic spin qubits into a three-dimensional metal–organic framework 140(38) (pp. 12090-12101) https://doi.org/10.1021/jacs.8b06733
- Chen et al. (2019) Folic acid-nanoscale gadolinium-porphyrin metal–organic frameworks: fluorescence and magnetic resonance dual-modality imaging and photodynamic therapy in hepatocellular carcinoma (pp. 57-74) https://doi.org/10.2147/IJN.S177880
- Kim et al. (2019) MOF× biopolymer: collaborative combination of metal–organic framework and biopolymer for advanced anticancer therapy 11(31) (pp. 27512-27520) https://doi.org/10.1021/acsami.9b05736
- Charbgoo et al. (2018) MUC1 aptamer-targeted DNA micelles for dual tumor therapy using doxorubicin and KLA peptide 14(3) (pp. 685-697) https://doi.org/10.1016/j.nano.2017.12.010
- Liu et al. (2017) Zirconium-based nanoscale metal–organic framework/poly (ε-caprolactone) mixed-matrix membranes as effective antimicrobials 9(47) (pp. 41512-41520) https://doi.org/10.1021/acsami.7b15826
- Abdelhamid (2021) Biointerface between ZIF-8 and biomolecules and their applications 11(1) (pp. 8283-8297)
- Abdelhamid (2021) Zeolitic imidazolate frameworks (ZIF-8) for biomedical applications: a review 28(34) (pp. 7023-7075) https://doi.org/10.2174/0929867328666210608143703
- An et al. (2012) Metal-adeninate vertices for the construction of an exceptionally porous metal–organic framework 3(1) (pp. 1-6) https://doi.org/10.1038/ncomms1618
- McKinlay et al. (2010) BioMOFs: metal–organic frameworks for biological and medical applications 49(36) (pp. 6260-6266) https://doi.org/10.1002/anie.201000048
- Nie et al. (2020) FRET as a novel strategy to enhance the singlet oxygen generation of porphyrinic MOF decorated self-disinfecting fabrics https://doi.org/10.1016/j.cej.2020.125012
- Orellana-Tavra et al. (2015) Amorphous metal–organic frameworks for drug delivery 51(73) (pp. 13878-13881)
- Lázaro et al. (2021) The excellent biocompatibility and negligible immune response of the titanium heterometallic MOF MUV-10 9(31) (pp. 6144-6148) https://doi.org/10.1039/D1TB00981H
- Park et al. (2018) 3D long-range triplet migration in a water-stable metal–organic framework for upconversion-based ultralow-power in vivo imaging 140(16) (pp. 5493-5499) https://doi.org/10.1021/jacs.8b01613
- Yan et al. (2021) Co (ii)-based metal–organic framework induces apoptosis through activating the HIF-1α/BNIP3 signaling pathway in microglial cells 8(10) (pp. 2866-2882) https://doi.org/10.1039/D1EN00719J
- Horcajada et al. (2010) Porous metal–organic-framework nanoscale carriers as a potential platform for drug delivery and imaging 9(2) (pp. 172-178) https://doi.org/10.1038/nmat2608
- Zhu et al. (2018) Versatile surface functionalization of metal–organic frameworks through direct metal coordination with a phenolic lipid enables diverse applications 28(16) https://doi.org/10.1002/adfm.201705274
- Cui et al. (2021) Outstanding drug-loading/release capacity of hollow Fe-metal–organic framework-based microcapsules: a potential multifunctional drug-delivery platform 60(3) (pp. 1664-1671) https://doi.org/10.1021/acs.inorgchem.0c03156
- Sun et al. (2021) Dual functions of pH-sensitive cation Zr-MOF for 5-Fu: large drug-loading capacity and high-sensitivity fluorescence detection 50(30) (pp. 10524-10532) https://doi.org/10.1039/D1DT01772A
- Nirosha Yalamandala et al. (2021) Advances in functional metal–organic frameworks based on-demand drug delivery systems for tumor therapeutics 1(8) https://doi.org/10.1002/anbr.202100014
- Lan et al. (2019) Nanoscale metal–organic frameworks for phototherapy of cancer (pp. 65-81) https://doi.org/10.1016/j.ccr.2017.09.007
- Pereira et al. (2016) Porphyrin-based metal–organic frameworks as heterogeneous catalysts in oxidation reactions 21(10) https://doi.org/10.3390/molecules21101348
- Sun et al. (2016) An in situ one-pot synthetic approach towards multivariate zirconium MOFs 128(22) (pp. 6581-6585) https://doi.org/10.1002/ange.201602274
- Zhang et al. (2021) Development of an ionic porphyrin-based platform as a biomimetic light-harvesting agent for high-performance photoenzymatic synthesis of methanol from CO2 9(34) (pp. 11503-11511) https://doi.org/10.1021/acssuschemeng.1c03737
- He et al. (2021) 5, 10, 15, 20-tetrakis (4-carboxylphenyl) porphyrin functionalized NiCo2S4 yolk-shell nanospheres: excellent peroxidase-like activity, catalytic mechanism and fast cascade colorimetric biosensor for cholesterol https://doi.org/10.1016/j.snb.2020.128850
- Kong et al. (2021) In situ porphyrin substitution in a Zr (IV)-MOF for stability enhancement and photocatalytic CO2 reduction 17(22) https://doi.org/10.1002/smll.202005357
- Mathew and Sujatha (2021) Interactions of porphyrins with DNA: a review focusing recent advances in chemical modifications on porphyrins as artificial nucleases https://doi.org/10.1016/j.jinorgbio.2021.111434
- Feng et al. (2014) A highly stable porphyrinic zirconium metal–organic framework with shp-a topology 136(51) (pp. 17714-17717) https://doi.org/10.1021/ja510525s
- Paolesse et al. (2017) Porphyrinoids for chemical sensor applications 117(4) (pp. 2517-2583) https://doi.org/10.1021/acs.chemrev.6b00361
- O’Connor et al. (2009) Porphyrin and nonporphyrin photosensitizers in oncology: preclinical and clinical advances in photodynamic therapy 85(5) (pp. 1053-1074) https://doi.org/10.1111/j.1751-1097.2009.00585.x
- Rodrigues et al. (2018) Photodynamic therapy at low-light fluence rate: In vitro assays on colon cancer cells 25(1) (pp. 1-6) https://doi.org/10.1109/JSTQE.2018.2889426
- Tsolekile et al. (2019) Porphyrin as diagnostic and therapeutic agent 24(14) https://doi.org/10.3390/molecules24142669
- Rotenberg and Margalit (1985) Deuteroporphyrin-albumin binding equilibrium. The effects of porphyrin self-aggregation studied for the human and the bovine proteins 229(1) (pp. 197-203) https://doi.org/10.1042/bj2290197
- Kano et al. (1987) Self aggregation of cationic porphyrin in water 60(4) (pp. 1281-1287) https://doi.org/10.1246/bcsj.60.1281
- Hachimine et al. (2007) Sonodynamic therapy of cancer using a novel porphyrin derivative, DCPH-P-Na (I), which is devoid of photosensitivity 98(6) (pp. 916-920) https://doi.org/10.1111/j.1349-7006.2007.00468.x
- Cheng et al. (2016) An O2 self-sufficient biomimetic nanoplatform for highly specific and efficient photodynamic therapy 26(43) (pp. 7847-7860) https://doi.org/10.1002/adfm.201603212
- Liu et al. (2017) Multifunctional metal–organic framework nanoprobe for cathepsin B-activated cancer cell imaging and chemo-photodynamic therapy 9(3) (pp. 2150-2158) https://doi.org/10.1021/acsami.6b14446
- Chen et al. (2021) Metal–organic framework-based nanoagents for effective tumor therapy by dual dynamics-amplified oxidative stress 13(38) (pp. 45201-45213) https://doi.org/10.1021/acsami.1c11032
- Yu et al. (2021) Silk fibroin-capped metal–organic framework for tumor-specific redox dyshomeostasis treatment synergized by deoxygenation-driven chemotherapy (pp. 545-560) https://doi.org/10.1016/j.actbio.2021.11.009
- Zhao et al. (2021) Tailoring aggregation extent of photosensitizer to boost phototherapy potency for eliciting systemic antitumor immunity 34(8) https://doi.org/10.1002/adma.202106390
- Huang et al. (2019) NIR-activated “OFF/ON” Photodynamic therapy by a hybrid nanoplatform with upper critical solution temperature block copolymers and gold nanorods 20(10) (pp. 3873-3883) https://doi.org/10.1021/acs.biomac.9b00963
- Tian et al. (2019) GSH-activated MRI-guided enhanced photodynamic-and chemo-combination therapy with a MnO2-coated porphyrin metal organic framework 55(44) (pp. 6241-6244)
- Lismont et al. (2017) Metal–organic framework nanoparticles in photodynamic therapy: current status and perspectives 27(14) https://doi.org/10.1002/adfm.201606314
- Judzewitsch et al. (2021) Photo-enhanced antimicrobial activity of polymers containing an embedded photosensitiser 133(45) (pp. 24450-24458) https://doi.org/10.1002/ange.202110672
- Zeng et al. (2018) π-extended benzoporphyrin-based metal–organic framework for inhibition of tumor metastasis 12(5) (pp. 4630-4640) https://doi.org/10.1021/acsnano.8b01186
- Lo et al. (2020) The unique features and promises of phthalocyanines as advanced photosensitisers for photodynamic therapy of cancer 49(4) (pp. 1041-1056) https://doi.org/10.1039/C9CS00129H
- Babu et al. (2020) Sn (iv) N-confused porphyrins as photosensitizer dyes for photodynamic therapy in the near IR region 49(43) (pp. 15180-15183) https://doi.org/10.1039/D0DT03296D
- Guo et al. (2018) Selective visible-light-driven oxygen reduction to hydrogen peroxide using BODIPY photosensitizers 54(7) (pp. 845-848)
- Montoya et al. (2005) Natural anthraquinones probed as Type I and Type II photosensitizers: singlet oxygen and superoxide anion production 78(1) (pp. 77-83) https://doi.org/10.1016/j.jphotobiol.2004.09.009
- Hadjur, C., Wagnières, G., Ihringer, F., Monnier, P., van den Bergh, H.: Production of the free radicals O
- .-
- 2
- and
- .
- OH by irradiation of the photosensitizer zinc (II) phthalocyanine. J. Photochem. Photobiol. B Biol.
- 38
- (2–3), 196–202 (1997)
- Nardin et al. (2019) Photosensitizer activation drives apoptosis by interorganellar Ca2+ transfer and superoxide production in bystander cancer cells 8(10) https://doi.org/10.3390/cells8101175
- Baier et al. (2006) Singlet oxygen generation by UVA light exposure of endogenous photosensitizers 91(4) (pp. 1452-1459) https://doi.org/10.1529/biophysj.106.082388
- Tsay et al. (2007) Singlet oxygen production by peptide-coated quantum dot− photosensitizer conjugates 129(21) (pp. 6865-6871) https://doi.org/10.1021/ja070713i
- Wang et al. (2018) Highly efficient photosensitizers with far-red/near-infrared aggregation-induced emission for in vitro and in vivo cancer theranostics 30(39) https://doi.org/10.1002/adma.201802105
- Wang et al. (2019) Cancer-cell-activated photodynamic therapy assisted by Cu (II)-based metal–organic framework 13(6) (pp. 6879-6890) https://doi.org/10.1021/acsnano.9b01665
- Abrahams et al. (1991) A new type of infinite 3D polymeric network containing 4-connected, peripherally-linked metalloporphyrin building blocks 113(9) (pp. 3606-3607) https://doi.org/10.1021/ja00009a065
- Muniappan et al. (2007) Porphyrin framework solids. Synthesis and structure of hybrid coordination polymers of tetra (carboxyphenyl) porphyrins and lanthanide-bridging ions 46(14) (pp. 5544-5554) https://doi.org/10.1021/ic0701099
- Qin et al. (2016) Derivation and decoration of nets with trigonal-prismatic nodes: a unique route to reticular synthesis of metal–organic frameworks 138(16) (pp. 5299-5307) https://doi.org/10.1021/jacs.6b01093
- Huang et al. (2019) Disclosing CO2 activation mechanism by hydroxyl-induced crystalline structure transformation in electrocatalytic process 1(6) (pp. 1656-1668) https://doi.org/10.1016/j.matt.2019.07.003
- Wang et al. (2019) Record high cationic dye separation performance for water sanitation using a neutral coordination framework 7(9) (pp. 4751-4758) https://doi.org/10.1039/C8TA12092G
- Dong et al. (2019) A highly ruffled distorted nickel-imidazolylporphyrin framework with 1D open nano-sized channels (pp. 14-18) https://doi.org/10.1016/j.inoche.2019.03.034
- Suslick et al. (2005) Microporous porphyrin solids 38(4) (pp. 283-291) https://doi.org/10.1021/ar040173j
- Cao et al. (2019) Porphyrinic silver cluster assembled material for simultaneous capture and photocatalysis of mustard-gas simulant 141(37) (pp. 14505-14509) https://doi.org/10.1021/jacs.9b05952
- Lu et al. (2014) Nanoscale metal–organic framework for highly effective photodynamic therapy of resistant head and neck cancer 136(48) (pp. 16712-16715) https://doi.org/10.1021/ja508679h
- Rieter et al. (2006) Nanoscale metal− organic frameworks as potential multimodal contrast enhancing agents 128(28) (pp. 9024-9025) https://doi.org/10.1021/ja0627444
- Simon-Yarza et al. (2018) Nanoparticles of metal–organic frameworks: on the road to in vivo efficacy in biomedicine 30(37) https://doi.org/10.1002/adma.201707365
- Arun Kumar et al. (2021) Two-dimensional metal organic frameworks for biomedical applications 13(2) https://doi.org/10.1002/wnan.1674
- Beg et al. (2017) Nanoporous metal organic frameworks as hybrid polymer–metal composites for drug delivery and biomedical applications 22(4) (pp. 625-637) https://doi.org/10.1016/j.drudis.2016.10.001
- Jiang et al. (2021) Bioinspired adhesive and tumor microenvironment responsive nanoMOFs assembled 3D-printed scaffold for anti-tumor therapy and bone regeneration https://doi.org/10.1016/j.nantod.2021.101182
- Huang et al. (2021) Curcumin-loaded nanoMOFs@ CMFP: a biological preserving paste with antibacterial properties and long-acting, controllable release https://doi.org/10.1016/j.foodchem.2020.127987
- Qiu et al. (2021) Porous nanoparticles with engineered shells release their drug cargo in cancer cells https://doi.org/10.1016/j.ijpharm.2021.121230
- Li et al. (2017) Composite CD-MOF nanocrystals-containing microspheres for sustained drug delivery 9(22) (pp. 7454-7463) https://doi.org/10.1039/C6NR07593B
- Xu et al. (2020) Highly stable and biocompatible hyaluronic acid-rehabilitated nanoscale MOF-Fe2+ induced ferroptosis in breast cancer cells 8(39) (pp. 9129-9138) https://doi.org/10.1039/D0TB01616K
- Della Rocca et al. (2011) Nanoscale metal–organic frameworks for biomedical imaging and drug delivery 44(10) (pp. 957-968) https://doi.org/10.1021/ar200028a
- Flügel et al. (2012) Synthetic routes toward MOF nanomorphologies 22(20) (pp. 10119-10133) https://doi.org/10.1039/c2jm15675j
- Liu et al. (2016) Nanoscale metal− organic frameworks for combined photodynamic and radiation therapy in cancer treatment (pp. 1-9) https://doi.org/10.1016/j.biomaterials.2016.04.034
- Miller et al. (2010) Biodegradable therapeutic MOFs for the delivery of bioactive molecules 46(25) (pp. 4526-4528)
- Sharma et al. (2019) Copper-gallic acid nanoscale metal–organic framework for combined drug delivery and photodynamic therapy 2(5) (pp. 2092-2101) https://doi.org/10.1021/acsabm.9b00116
- Yang et al. (2016) Nanoscale metal–organic particles with rapid clearance for magnetic resonance imaging-guided photothermal therapy 10(2) (pp. 2774-2781) https://doi.org/10.1021/acsnano.5b07882
- Zhang et al. (2018) Theranostic Mn-porphyrin metal–organic frameworks for magnetic resonance imaging-guided nitric oxide and photothermal synergistic therapy 10(34) (pp. 28390-28398) https://doi.org/10.1021/acsami.8b09680
- Zhang et al. (2015) A porphyrin photosensitized metal–organic framework for cancer cell apoptosis and caspase responsive theranostics 51(54) (pp. 10831-10834)
- Rabiee et al. (2020) Recent advances in porphyrin-based nanocomposites for effective targeted imaging and therapy https://doi.org/10.1016/j.biomaterials.2019.119707
- Gao et al. (2014) Metal–metalloporphyrin frameworks: a resurging class of functional materials 43(16) (pp. 5841-5866) https://doi.org/10.1039/C4CS00001C
- Chen et al. (2021) Porphyrin-based metal–organic frameworks for biomedical applications 60(10) (pp. 5010-5035) https://doi.org/10.1002/anie.201909880
- Xie et al. (2020) A singlet oxygen reservoir based on poly-pyridone and porphyrin nanoscale metal–organic framework for cancer therapy (pp. 1187-1202) https://doi.org/10.31635/ccschem.020.202000201
- Zhang et al. (2020) A historical perspective on porphyrin-based metal–organic frameworks and their applications https://doi.org/10.1016/j.ccr.2020.213615
- Vitillo and Bordiga (2017) Increasing the stability of Mg2(dobpdc) metal–organic framework in air through solvent removal 1(3) (pp. 444-448) https://doi.org/10.1039/C6QM00220J
- Shearer et al. (2013) In situ infrared spectroscopic and gravimetric characterisation of the solvent removal and dehydroxylation of the metal organic frameworks UiO-66 and UiO-67 56(9–10) (pp. 770-782) https://doi.org/10.1007/s11244-013-0027-0
- Ma et al. (2009) Highly porous and robust 4, 8-connected metal−organic frameworks for hydrogen storage 131(13) (pp. 4610-4612) https://doi.org/10.1021/ja809590n
- Tan et al. (2012) Tuning MOF stability and porosity via adding rigid pillars 51(18) (pp. 9649-9654) https://doi.org/10.1021/ic300778m
- Hönicke et al. (2018) Balancing mechanical stability and ultrahigh porosity in crystalline framework materials 57(42) (pp. 13780-13783) https://doi.org/10.1002/anie.201808240
- Farha et al. (2011) Active-site-accessible, Porphyrinic metal−organic framework materials 133(15) (pp. 5652-5655) https://doi.org/10.1021/ja111042f
- Fateeva et al. (2015) Iron and porphyrin metal–organic frameworks: insight into structural diversity, stability, and porosity 15(4) (pp. 1819-1826) https://doi.org/10.1021/cg501855k
- Dutta et al. (2018) Encapsulation of silver nanoparticles in an amine-functionalized porphyrin metal–organic framework and its use as a heterogeneous catalyst for CO2 fixation under atmospheric pressure 13(18) (pp. 2677-2684) https://doi.org/10.1002/asia.201800815
- Fateeva et al. (2011) Series of porous 3-D coordination polymers based on iron (III) and porphyrin derivatives 23(20) (pp. 4641-4651) https://doi.org/10.1021/cm2025747
- Mamardashvili et al. (2000) Solubility of alkylporphyrins 5(6) (pp. 762-766) https://doi.org/10.3390/50600762
- Deda et al. (2020) Porphyrin derivative nanoformulations for therapy and antiparasitic agents 25(9) https://doi.org/10.3390/molecules25092080
- Sobczyński et al. (2013) Influence of aqueous media properties on aggregation and solubility of four structurally related meso-porphyrin photosensitizers evaluated by spectrophotometric measurements 68(2) (pp. 100-109)
- Kano et al. (2000) Factors influencing self-aggregation tendencies of cationic porphyrins in aqueous solution 122(31) (pp. 7494-7502) https://doi.org/10.1021/ja000738g
- Ou et al. (2007) Preparation and optical properties of organic nanoparticles of porphyrin without self-aggregation 189(1) (pp. 7-14) https://doi.org/10.1016/j.jphotochem.2006.12.042
- dos Santos et al. (2013) Synthesis of functionalized chlorins sterically-prevented from self-aggregation 99(2) (pp. 402-411) https://doi.org/10.1016/j.dyepig.2013.05.024
- Toncelli et al. (2013) Controlling the aggregation of 5, 10, 15, 20-tetrakis-(4-sulfonatophenyl)-porphyrin by the use of polycations derived from polyketones bearing charged aromatic groups 98(1) (pp. 51-63) https://doi.org/10.1016/j.dyepig.2013.01.008
- Konishi et al. (2003) Improvement of quantum yields for photoinduced energy/electron transfer by isolation of self-aggregative zinc tetraphenyl porphyrin-pendant polymer using cyclodextrin inclusion in aqueous solution 107(41) (pp. 11261-11266) https://doi.org/10.1021/jp0273878
- Lv et al. (2017) A base-resistant metalloporphyrin metal–organic framework for C-H bond halogenation 139(1) (pp. 211-217) https://doi.org/10.1021/jacs.6b09463
- Zhang et al. (2018) Nanozyme decorated metal–organic frameworks for enhanced photodynamic therapy 12(1) (pp. 651-661) https://doi.org/10.1021/acsnano.7b07746
- Enakieva et al. (2019) Highly proton-conductive zinc metal–organic framework based on nickel (II) porphyrinylphosphonate 25(45) (pp. 10552-10556) https://doi.org/10.1002/chem.201902212
- Le Gac et al. (2019) Hg (II)-mediated Tl (I)-to-Tl (III) oxidation in dynamic Pb (II)/Tl porphyrin complexes 25(3) (pp. 845-853) https://doi.org/10.1002/chem.201804713
- ZareKarizi et al. (2018) Pillar-layered MOFs: functionality, interpenetration, flexibility and applications 6(40) (pp. 19288-19329) https://doi.org/10.1039/C8TA03306D
- Sakuma et al. (2016) Control of local structures and photophysical properties of zinc porphyrin-based supramolecular assemblies structurally organized by regioselective ligand coordination 18(7) (pp. 5453-5463) https://doi.org/10.1039/C5CP07110K
- Barron et al. (2010) A bioinspired synthetic approach for building metal− organic frameworks with accessible metal centers 49(22) (pp. 10217-10219) https://doi.org/10.1021/ic101459j
- Lee et al. (2011) Light-harvesting metal–organic frameworks (MOFs): efficient strut-to-strut energy transfer in bodipy and porphyrin-based MOFs 133(40) (pp. 15858-15861) https://doi.org/10.1021/ja206029a
- Xie et al. (2013) Highly efficient C–H oxidative activation by a porous MnIII–porphyrin metal–organic framework under mild conditions 19(42) (pp. 14316-14321) https://doi.org/10.1002/chem.201302025
- Mishra et al. (2019) Structural diversity in tetrakis (4-pyridyl) porphyrin supramolecular building blocks 19(6) (pp. 3529-3542) https://doi.org/10.1021/acs.cgd.9b00399
- Johnson et al. (2016) A new approach to non-coordinating anions: lewis acid enhancement of porphyrin metal centers in a zwitterionic metal–organic framework 138(32) (pp. 10293-10298) https://doi.org/10.1021/jacs.6b05626
- Maares et al. (2019) Alkali phosphonate metal–organic frameworks 25(48) (pp. 11214-11217) https://doi.org/10.1002/chem.201902207
- Fang et al. (2019) Electrochemical, spectroelectrochemical, and structural studies of mono-and diphosphorylated zinc porphyrins and their self-assemblies 58(7) (pp. 4665-4678) https://doi.org/10.1021/acs.inorgchem.9b00268
- Shmilovits et al. (2004) Coordination polymers of tetra (4-carboxyphenyl) porphyrins sustained by tetrahedral zinc ion linkers 4(3) (pp. 633-638) https://doi.org/10.1021/cg0342009
- Diskin-Posner and Goldberg (2001) Porphyrin sieves. Designing open networks of tetra (carboxyphenyl) porphyrins by extended coordination through sodium ion auxiliaries 25(7) (pp. 899-904) https://doi.org/10.1039/b100580b
- Kosal et al. (2002) A calcium-bridged porphyrin coordination network 6(06) (pp. 377-381) https://doi.org/10.1142/S1088424602000464
- Diskin-Posner et al. (2000) Crystal engineering of metalloporphyrin zeolite analogues 112(7) (pp. 1344-1348) https://doi.org/10.1002/(SICI)1521-3757(20000403)112:7<1344::AID-ANGE1344>3.0.CO;2-8
- Diskin-Posner et al. (2000) New effective synthons for supramolecular self-assembly of meso-carboxyphenylporphyrins (pp. 585-586)
- Diskin-Posner et al. (2001) Crystal engineering of 2-D and 3-D multiporphyrin architectures—the versatile topologies of tetracarboxyphenylporphyrin-based materials 2001(10) (pp. 2515-2523) https://doi.org/10.1002/1099-0682(200109)2001:10<2515::AID-EJIC2515>3.0.CO;2-0
- Shmilovits et al. (2003) Crystal engineering of “porphyrin sieves” based on coordination polymers of Pd-and Pt-tetra (4-carboxyphenyl) porphyrin 3(5) (pp. 855-863) https://doi.org/10.1021/cg034071w
- Zou et al. (2012) Five porphyrin-core-dependent metal–organic frameworks and framework-dependent fluorescent properties 14(14) (pp. 4850-4856) https://doi.org/10.1039/c2ce25357g
- Chen (2012) Hydrothermal preparation, crystal structure and properties of {[ZnTCPP (EtOH)][Zn (en)]2}n (EtOH)2n with a novel two-dimensional (2-D) Motif 30(2) (pp. 273-276) https://doi.org/10.1002/cjoc.201180485
- Amayuelas et al. (2017) Cationic Mn2+/H+ exchange leading a slow solid-state transformation of a 2D porphyrinic network at ambient conditions (pp. 161-167) https://doi.org/10.1016/j.jssc.2017.01.012
- George et al. (2006) Porphyrin supramolecular solids assembled with the aid of lanthanide ions 6(12) (pp. 2651-2654) https://doi.org/10.1021/cg060520r
- Lipstman and Goldberg (2008) 2D and 3D coordination networks of tetra (carboxyphenyl)-porphyrins with cerium and thulium ions 890(1–3) (pp. 101-106) https://doi.org/10.1016/j.molstruc.2008.03.044
- George, S., Goldberg, I.: Crystal structure of catena-tris (5, 10, 15, 20-(4-carboxylatophenyl)-porphyrin)-aqua-tetradysprosium-trizinc solvate, (C
- 48
- H
- 24
- N
- 4
- O
- 8
- Zn)
- 3
- Dy4 (H
- 2
- O)·(solvent)
- x
- . Z. Krist.-New Cryst. St.
- 226
- (3),411–413 (2011)
- Rhauderwiek et al. (2017) Co-ligand dependent formation and phase transformation of four porphyrin-based cerium metal–organic frameworks 17(6) (pp. 3462-3474) https://doi.org/10.1021/acs.cgd.7b00450
- Choi et al. (2009) Highly tunable metal–organic frameworks with open metal centers 11(4) (pp. 553-555) https://doi.org/10.1039/B819707P
- Makiura et al. (2014) Porphyrin-based coordination polymer composed of layered pillarless two-dimensional networks 43(7) (pp. 1161-1163) https://doi.org/10.1246/cl.140275
- Gallagher et al. (2016) Dioxygen binding at a four-coordinate cobaltous porphyrin site in a metal–organic framework: structural, EPR, and O2 adsorption analysis 3(4) (pp. 536-540) https://doi.org/10.1039/C5QI00275C
- Anderson et al. (2014) A five-coordinate heme dioxygen adduct isolated within a metal–organic framework 136(47) (pp. 16489-16492) https://doi.org/10.1021/ja5103103
- Gallagher et al. (2017) CO binding at a four-coordinate cobaltous porphyrin site in a metal–organic framework: structural, EPR, and Gas Adsorption Analysis 56(8) (pp. 4654-4661) https://doi.org/10.1021/acs.inorgchem.7b00292
- Dolgopolova et al. (2015) A bio-inspired approach for chromophore communication: ligand-to-ligand and host-to-guest energy transfer in hybrid crystalline scaffolds 54(46) (pp. 13639-13643) https://doi.org/10.1002/anie.201507400
- Park et al. (2017) Efficient energy transfer (EnT) in pyrene-and porphyrin-based mixed-ligand metal–organic frameworks 9(44) (pp. 38670-38677) https://doi.org/10.1021/acsami.7b14135
- Yang et al. (2019) Hierarchical hybrid metal–organic frameworks: tuning the visible/near-infrared optical properties by a combination of porphyrin and its isomer units 58(7) (pp. 4647-4656) https://doi.org/10.1021/acs.inorgchem.9b00251
- Lu et al. (2018) Low-dose X-ray radiotherapy–radiodynamic therapy via nanoscale metal–organic frameworks enhances checkpoint blockade immunotherapy 2(8) (pp. 600-610) https://doi.org/10.1038/s41551-018-0203-4
- Xu et al. (2018) Isolated π-interaction sites in mesoporous MOF backbone for repetitive and reversible dynamics in water 11(1) (pp. 973-981) https://doi.org/10.1021/acsami.8b19211
- Tripuramallu et al. (2019) Location controlled symmetry reduction: paradigm of an open metalloporphyrin framework based on the tetracarboxy porphyrin linker 21(35) (pp. 5216-5221) https://doi.org/10.1039/C9CE01107B
- Rönfeldt et al. (2020) Scandium metal–organic frameworks containing tetracarboxylate linker molecules: synthesis, structural relationships, and properties 20(7) (pp. 4686-4694) https://doi.org/10.1021/acs.cgd.0c00478
- Barron et al. (2009) Highly tunable heterometallic frameworks constructed from paddle-wheel units and metalloporphyrins 9(4) (pp. 1960-1965) https://doi.org/10.1021/cg801267m
- Martinez-Bulit et al. (2019) Solvent and steric influences on rotational dynamics in porphyrinic metal–organic frameworks with mechanically interlocked pillars 19(10) (pp. 5679-5685) https://doi.org/10.1021/acs.cgd.9b00669
- Zhang et al. (2018) Pore-environment engineering with multiple metal sites in rare-earth porphyrinic metal–organic frameworks 57(18) (pp. 5095-5099) https://doi.org/10.1002/anie.201802661
- Lipstman et al. (2007) Framework coordination polymers of tetra (4-carboxyphenyl) porphyrin and lanthanide ions in crystalline solids (pp. 3273-3281) https://doi.org/10.1039/b703698a
- Choi et al. (2009) Pillared porphyrin homologous series: intergrowth in metal− organic frameworks 48(2) (pp. 426-428) https://doi.org/10.1021/ic801677y
- Ding et al. (2017) Controlled intercalation and chemical exfoliation of layered metal–organic frameworks using a chemically labile intercalating agent 139(27) (pp. 9136-9139) https://doi.org/10.1021/jacs.7b04829
- Burnett et al. (2011) Stepwise synthesis of metal–organic frameworks: replacement of structural organic linkers 133(26) (pp. 9984-9987) https://doi.org/10.1021/ja201911v
- Planes et al. (2020) Incorporation of clathrochelate-based metalloligands in metal–organic frameworks by solvent-assisted ligand exchange 20(3) (pp. 1394-1399) https://doi.org/10.1021/acs.cgd.9b01697
- Liu et al. (2020) A series of highly stable porphyrinic metal–organic frameworks based on iron–oxo chain clusters: design, synthesis and biomimetic catalysis 8(17) (pp. 8376-8382) https://doi.org/10.1039/D0TA02033H
- Nefedov et al. (2019) Coordination self-assembly through weak interactions in meso-dialkoxyphosphoryl-substituted zinc porphyrinates 48(16) (pp. 5372-5383) https://doi.org/10.1039/C9DT00706G
- Mitrofanov et al. (2019) Facile synthesis and self-assembly of zinc (2-Diethoxyphosphorylethynyl) porphyrins 2019(10) (pp. 1313-1328) https://doi.org/10.1002/ejic.201900004
- Wang et al. (2016) Pyrazolate-based porphyrinic metal–organic framework with extraordinary base-resistance 138(3) (pp. 914-919) https://doi.org/10.1021/jacs.5b10881
- Lu et al. (2015) A chlorin-based nanoscale metal–organic framework for photodynamic therapy of colon cancers 137(24) (pp. 7600-7603) https://doi.org/10.1021/jacs.5b04069
- Feng et al. (2019) Elucidating J-aggregation effect in boosting singlet-oxygen evolution using zirconium-porphyrin frameworks: a comprehensive structural, catalytic, and spectroscopic study 11(48) (pp. 45118-45125) https://doi.org/10.1021/acsami.9b17569
- He et al. (2019) A versatile metalloporphyrinic framework platform for highly efficient bioinspired, photo-and asymmetric catalysis 58(1) (pp. 168-172) https://doi.org/10.1002/anie.201810294
- Abdulaeva et al. (2019) Imidazoporphyrins as supramolecular tectons: synthesis and self-assembly of zinc 2-(4-pyridyl)-1 H-imidazo [4, 5-b] porphyrinate 21(9) (pp. 1488-1498) https://doi.org/10.1039/C8CE01992D
- Dhamija et al. (2019) Molecule to supramolecule: chirality induction, inversion, and amplification in a Mg (II) porphyrin Dimer templated by Chiral Diols 59(1) (pp. 801-809) https://doi.org/10.1021/acs.inorgchem.9b03062
- Battistin et al. (2020) Orthogonal coordination chemistry of PTA toward Ru (II) and Zn (II)(PTA= 1, 3, 5-Triaza-7-phosphaadamantane) for the construction of 1D and 2D metal-mediated porphyrin networks 59(6) (pp. 4068-4079) https://doi.org/10.1021/acs.inorgchem.0c00080
- Niu et al. (2019) Morphology-dependent third-order optical nonlinearity of a 2D Co-based metal–organic framework with a porphyrinic skeleton 55(33) (pp. 4873-4876)
- Wan et al. (2018) ROS-induced NO generation for gas therapy and sensitizing photodynamic therapy of tumor (pp. 51-62) https://doi.org/10.1016/j.biomaterials.2018.09.004
- Zhou et al. (2019) Porphyrin–palladium hydride MOF nanoparticles for tumor-targeting photoacoustic imaging-guided hydrogenothermal cancer therapy 4(5) (pp. 1185-1193) https://doi.org/10.1039/C9NH00021F
- Luo et al. (2019) Light-Induced redox-responsive smart drug delivery system by using selenium-containing polymer@ MOF shell/core nanocomposite 8(15) https://doi.org/10.1002/adhm.201900406
- Chun et al. (2020) PCN-223 as a drug carrier for potential treatment of colorectal cancer (pp. 290-296) https://doi.org/10.1016/j.jiec.2020.01.010
- Shahriari et al. (2019) Synthesis of hyaluronic acid-based polymersomes for doxorubicin delivery to metastatic breast cancer https://doi.org/10.1016/j.ijpharm.2019.118835
- Bagheri et al. (2020) Targeted doxorubicin-loaded mesenchymal stem cells-derived exosomes as a versatile platform for fighting against colorectal cancer https://doi.org/10.1016/j.lfs.2020.118369
- Awwad and Angkawinitwong (2018) Overview of antibody drug delivery 10(3) https://doi.org/10.3390/pharmaceutics10030083
- Araste et al. (2018) Peptide-based targeted therapeutics: focus on cancer treatment (pp. 141-162) https://doi.org/10.1016/j.jconrel.2018.11.004
- Alibolandi et al. (2016) Dextran-poly lactide-co-glycolide polymersomes decorated with folate-antennae for targeted delivery of docetaxel to breast adenocarcinima in vitro and in vivo (pp. 45-56) https://doi.org/10.1016/j.jconrel.2016.09.012
- Zhang et al. (2019) DNA-functionalized metal–organic framework: cell imaging, targeting drug delivery and photodynamic therapy 58(10) (pp. 6593-6596) https://doi.org/10.1021/acs.inorgchem.9b00734
- Lin et al. (2016) A porphyrin-based metal–organic framework as a pH-responsive drug carrier (pp. 307-312) https://doi.org/10.1016/j.jssc.2016.02.040
- Wang et al. (2019) The application of methylprednisolone nanoscale zirconium-porphyrin metal–organic framework (MPS-NPMOF) in the treatment of photoreceptor degeneration https://doi.org/10.2147/IJN.S225992
- Li et al. (2017) Cancer cell membrane camouflaged cascade bioreactor for cancer targeted starvation and photodynamic therapy 11(7) (pp. 7006-7018) https://doi.org/10.1021/acsnano.7b02533
- Zhao et al. (2019) Postsynthetic ligand exchange of metal–organic framework for photodynamic therapy 11(8) (pp. 7884-7892) https://doi.org/10.1021/acsami.9b00740
- He et al. (2020) Solvent-assisted self-assembly of a metal–organic framework based biocatalyst for cascade reaction driven photodynamic therapy 142(14) (pp. 6822-6832) https://doi.org/10.1021/jacs.0c02497
- Li et al. (2020) Controllable synthesis of Ni/Co-TCPP MOFs with different morphologies and their application in electrochemical detection of glucose 167(12) https://doi.org/10.1149/1945-7111/abac2a
- Zhao et al. (2020) Metal–organic frameworks with enhanced photodynamic therapy: synthesis, erythrocyte membrane camouflage, and aptamer-targeted aggregation 12(21) (pp. 23697-23706) https://doi.org/10.1021/acsami.0c04363
- Wang et al. (2019) Exploiting single atom iron centers in a porphyrin-like MOF for efficient cancer phototherapy 11(38) (pp. 35228-35237) https://doi.org/10.1021/acsami.9b11238
- Li et al. (2017) Heterodimers made of upconversion nanoparticles and metal–organic frameworks 139(39) (pp. 13804-13810) https://doi.org/10.1021/jacs.7b07302
- Zhou et al. (2018) One-pot synthetic approach toward porphyrinatozinc and heavy-atom involved Zr-NMOF and its application in photodynamic therapy 57(6) (pp. 3169-3176) https://doi.org/10.1021/acs.inorgchem.7b03204
- Li et al. (2018) A biomimetic theranostic O2-meter for cancer targeted photodynamic therapy and phosphorescence imaging (pp. 1-12) https://doi.org/10.1016/j.biomaterials.2017.10.021
- Liu et al. (2019) Nanozymes-engineered metal–organic frameworks for catalytic cascades-enhanced synergistic cancer therapy 19(8) (pp. 5674-5682) https://doi.org/10.1021/acs.nanolett.9b02253
- Zheng et al. (2018) Nanoscale mixed-component metal–organic frameworks with photosensitizer spatial-arrangement-dependent photochemistry for multimodal-imaging-guided photothermal therapy 30(19) (pp. 6867-6876) https://doi.org/10.1021/acs.chemmater.8b03043
- Hollingworth et al. (2000) The diagnostic and therapeutic impact of MRI: an observational multi-centre study 55(11) (pp. 825-831) https://doi.org/10.1053/crad.2000.0546
- Lee et al. (2004) Diagnostic CT scans: assessment of patient, physician, and radiologist awareness of radiation dose and possible risks 231(2) (pp. 393-398) https://doi.org/10.1148/radiol.2312030767
- De Ruysscher et al. (2012) PET scans in radiotherapy planning of lung cancer 75(2) (pp. 141-145) https://doi.org/10.1016/j.lungcan.2011.07.018
- Shung (2011) Diagnostic ultrasound: past, present, and future 31(6) (pp. 371-374) https://doi.org/10.5405/jmbe.871
- Zhang et al. (2019) Cell membrane-coated porphyrin metal–organic frameworks for cancer cell targeting and O2-evolving photodynamic therapy 11(43) (pp. 39594-39602) https://doi.org/10.1021/acsami.9b14084
- Liu et al. (2017) Fluorescent imaging-guided chemotherapy-and-photodynamic dual therapy with nanoscale porphyrin metal–organic framework 13(17) https://doi.org/10.1002/smll.201603459
- Liu et al. (2019) In situ polymerization on nanoscale metal–organic frameworks for enhanced physiological stability and stimulus-responsive intracellular drug delivery https://doi.org/10.1016/j.biomaterials.2019.119365
- Xia et al. (2020) Multi-modal channel cancer chemotherapy by 2D functional gadolinium metal–organic framework 8(7) https://doi.org/10.1093/nsr/nwaa221
- Liu et al. (2018) Hypoxia-triggered nanoscale metal–organic frameworks for enhanced anticancer activity 10(29) (pp. 24638-24647) https://doi.org/10.1021/acsami.8b07570
- He et al. (2019) Mn–porphyrin-based metal–organic framework with high longitudinal relaxivity for magnetic resonance imaging guidance and oxygen self-supplementing photodynamic therapy 11(45) (pp. 41946-41956) https://doi.org/10.1021/acsami.9b15083
- Wan et al. (2019) A Mn (III)-sealed metal–organic framework nanosystem for redox-unlocked tumor theranostics 13(6) (pp. 6561-6571) https://doi.org/10.1021/acsnano.9b00300
- Meng et al. (2018) Aptamer-functionalized nanoscale metal–organic frameworks for targeted photodynamic therapy 8(16) https://doi.org/10.7150/thno.26768
- Li et al. (2017) Cancer cell membrane-coated biomimetic platform for tumor targeted photodynamic therapy and hypoxia-amplified bioreductive therapy (pp. 149-161) https://doi.org/10.1016/j.biomaterials.2017.07.026
- Gao et al. (2020) A CD44-targeted Cu (ii) delivery 2D nanoplatform for sensitized disulfiram chemotherapy to triple-negative breast cancer 12(15) (pp. 8139-8146) https://doi.org/10.1039/D0NR00434K
- Hang et al. (2021) Controllable photodynamic performance via an acidic microenvironment based on two-dimensional metal–organic frameworks for photodynamic therapy (pp. 660-666) https://doi.org/10.1007/s12274-020-3093-1
- Zhang et al. (2018) Enhanced photodynamic therapy by reduced levels of intracellular glutathione obtained by employing a nano-MOF with cuii as the active center 130(18) (pp. 4985-4990) https://doi.org/10.1002/ange.201710800
- Shao et al. (2020) Engineering of upconverted metal–organic frameworks for near-infrared light-triggered combinational photodynamic/chemo-/immunotherapy against hypoxic tumors 142(8) (pp. 3939-3946) https://doi.org/10.1021/jacs.9b12788
- Han et al. (2020) Enhanced photocatalytic activity and photothermal effects of cu-doped metal–organic frameworks for rapid treatment of bacteria-infected wounds https://doi.org/10.1016/j.apcatb.2019.118248
- Qin et al. (2020) Ionic liquid induced highly dense assembly of porphyrin in MOF nanosheets for photodynamic therapy 49(48) (pp. 17772-17778) https://doi.org/10.1039/D0DT03031G
- Ni et al. (2019) Nanoscale metal–organic framework mediates radical therapy to enhance cancer immunotherapy 5(7) (pp. 1892-1913) https://doi.org/10.1016/j.chempr.2019.05.013
- Wang et al. (2019) Nanoscaled porphyrinic metal–organic framework for photodynamic/photothermal therapy of tumor 40(16–17) (pp. 2204-2210) https://doi.org/10.1002/elps.201900005
- Yin et al. (2019) Persistent regulation of tumor microenvironment via circulating catalysis of MnFe2O4@ metal–organic frameworks for enhanced photodynamic therapy 29(25) https://doi.org/10.1002/adfm.201901417
- Wang et al. (2018) Porphyrinic metal–organic framework PCN-224 nanoparticles for near-infrared-induced attenuation of aggregation and neurotoxicity of Alzheimer’s Amyloid-β peptide 10(43) (pp. 36615-36621) https://doi.org/10.1021/acsami.8b15452
- Wang et al. (2019) Specific generation of singlet oxygen through the russell mechanism in hypoxic tumors and GSH depletion by Cu-TCPP nanosheets for cancer therapy 58(29) (pp. 9846-9850) https://doi.org/10.1002/anie.201903981
- Leng, X., Huang, H., Wang, W., Sai, N., You, L., Yin, X., Ni, J.: Zirconium-Porphyrin PCN-222: pH-responsive Controlled Anticancer Drug Oridonin. Evid.-Based Complement. Alternat. Med.
- 2018
- , 3249023 (2018)
- Lin et al. (2019) A photodynamic system based on endogenous bioluminescence for in vitro anticancer studies 645(18–19) (pp. 1161-1164) https://doi.org/10.1002/zaac.201900144
- Wang et al. (2020) Fusiform-like copper (II)-based metal–organic framework through relief hypoxia and GSH-depletion co-enhanced starvation and chemodynamic synergetic cancer therapy 12(15) (pp. 17254-17267) https://doi.org/10.1021/acsami.0c01539
- Ning et al. (2018) Imparting designer biorecognition functionality to metal–organic frameworks by a DNA-mediated surface engineering strategy 14(11) https://doi.org/10.1002/smll.201703812
- Zheng et al. (2020) Integration of metal–organic framework with a photoactive porous-organic polymer for interface enhanced phototherapy https://doi.org/10.1016/j.biomaterials.2020.119792
- Schlachter et al. (2021) Porphyrin-containing MOFs and COFs as heterogeneous photosensitizers for singlet oxygen-based antimicrobial nanodevices 13(23) (pp. 26651-26672) https://doi.org/10.1021/acsami.1c05234
- Bi et al. (2022) Development of 3D porous Ag+ decorated PCN-222@ graphene oxide-chitosan foam adsorbent with antibacterial property for recovering U (VI) from seawater https://doi.org/10.1016/j.seppur.2021.119900
- Chen et al. (2020) Titanium incorporation into Zr-porphyrinic metal–organic frameworks with enhanced antibacterial activity against multidrug-resistant pathogens 16(7) https://doi.org/10.1002/smll.201906240
- Xu et al. (2021) Manganese porphyrin-based metal–organic framework for synergistic sonodynamic therapy and ferroptosis in hypoxic tumors 11(4) https://doi.org/10.7150/thno.45511
- Wang et al. (2019) DNA-functionalized metal–organic framework nanoparticles for intracellular delivery of proteins 141(6) (pp. 2215-2219) https://doi.org/10.1021/jacs.8b12705
- Cheng et al. (2019) Nanotherapeutics interfere with cellular redox homeostasis for highly improved photodynamic therapy https://doi.org/10.1016/j.biomaterials.2019.119500
- Wang et al. (2019) Renal-clearable porphyrinic metal–organic framework nanodots for enhanced photodynamic therapy 13(8) (pp. 9206-9217) https://doi.org/10.1021/acsnano.9b03531
- Abdelhamid and Mathew (2022) Cellulose–metal organic frameworks (CelloMOFs) hybrid materials and their multifaceted applications: a review https://doi.org/10.1016/j.ccr.2021.214263
- Chen et al. (2021) Vancomycin-functionalized porphyrinic metal–organic framework PCN-224 with enhanced antibacterial activity against Staphylococcus aureus 16(15) (pp. 2022-2026) https://doi.org/10.1002/asia.202100546
- Luo et al. (2019) Dual metal–organic framework heterointerface 5(9) (pp. 1591-1601) https://doi.org/10.1021/acscentsci.9b00639
- Luo et al. (2021) Enhanced photocatalytic and photothermal properties of ecofriendly metal–organic framework heterojunction for rapid sterilization https://doi.org/10.1016/j.cej.2020.126730
- Liu et al. (2021) Effect of topology on photodynamic sterilization of porphyrinic metal–organic frameworks 27(39) (pp. 10151-10159) https://doi.org/10.1002/chem.202100920
- Han et al. (2021) Photothermy-strengthened photocatalytic activity of polydopamine-modified metal–organic frameworks for rapid therapy of bacteria-infected wounds (pp. 83-95) https://doi.org/10.1016/j.jmst.2020.05.055
- Yu et al. (2021) Theory-screened MOF-based single-atom catalysts for facile and effective therapy of biofilm-induced periodontitis https://doi.org/10.1016/j.cej.2021.133279
- Zhang et al. (2020) Near infrared light-triggered metal ion and photodynamic therapy based on AgNPs/porphyrinic MOFs for tumors and pathogens elimination https://doi.org/10.1016/j.biomaterials.2020.120029
- Xu et al. (2021) Plasmon induced dual excited synergistic effect in Au/metal–organic frameworks composite for enhanced antibacterial therapy 9(46) (pp. 9606-9614) https://doi.org/10.1039/D1TB02141A
- Tang et al. (2019) Preparation and characterization of tebuconazole metal–organic framework-based microcapsules with dual-microbicidal activity (pp. 225-232) https://doi.org/10.1016/j.cej.2018.11.147
- Min et al. (2021) Electrospun pullulan/PVA nanofibers integrated with thymol-loaded porphyrin metal− organic framework for antibacterial food packaging https://doi.org/10.1016/j.carbpol.2021.118391
- Zhang et al. (2021) The fluorescence imaging and precise suppression of bacterial infections in chronic wounds by porphyrin-based metal–organic framework nanorods 9(38) (pp. 8048-8055) https://doi.org/10.1039/D1TB01649K
- Wang et al. (2021) Graphene-like MOF nanosheets stabilize graphene oxide membranes enabling selective molecular sieving https://doi.org/10.1016/j.memsci.2021.119397
- Zhang et al. (2021) Integrating porphyrinic metal–organic frameworks in nanofibrous carrier for photodynamic antimicrobial application 13(22) https://doi.org/10.3390/polym13223942
- Nie et al. (2021) PCN-224 nanoparticle/polyacrylonitrile nanofiber membrane for light-driven bacterial inactivation 11(12) https://doi.org/10.3390/nano11123162
- Tang et al. (2021) Preparation of a porphyrin metal–organic framework with desirable photodynamic antimicrobial activity for sustainable plant disease management 69(8) (pp. 2382-2391) https://doi.org/10.1021/acs.jafc.0c06487
- Ximing et al. (2017) Preparation of spherical metal–organic frameworks encapsulating Ag nanoparticles and study on its antibacterial activity (pp. 698-707) https://doi.org/10.1016/j.msec.2017.07.027
- Mao et al. (2021) Rugby-ball like Ag modified zirconium porphyrin metal–organic frameworks nanohybrid for antimicrobial activity: synergistic effect for significantly enhancing photoactivation capacity https://doi.org/10.1016/j.colsurfa.2020.125888
- Deng et al. (2019) Porphyrin MOF dots-based, function-adaptive nanoplatform for enhanced penetration and photodynamic eradication of bacterial biofilms 29(30) https://doi.org/10.1002/adfm.201903018
- Lan et al. (2017) Nanoscale metal–organic layers for deeply penetrating X-ray-induced photodynamic therapy 129(40) (pp. 12270-12274) https://doi.org/10.1002/ange.201704828
- Teo et al. (2021) Facile preparation of antibacterial MOF-fabric systems for functional protective wearables 2(4) (pp. 567-578) https://doi.org/10.1002/smm2.1046

 10.1007/s40097-022-00500-6
10.1007/s40097-022-00500-6