Encapsulating MoS2-nanoflowers conjugated with chitosan oligosaccharide into electrospun nanofibrous scaffolds for photothermal inactivation of bacteria
- Center for Global Health, The Key Laboratory of Modern Toxicology, Ministry of Education, School of Public Health, Nanjing Medical University, Nanjing, 211166, CN
- State Key Laboratory of Nuclear Resources and Environment, School of Nuclear Science and Engineering, East China University of Technology, Nanchang, 330013, CN
- School of Chemistry and Chemical Engineering, Southeast University, Nanjing, 211189, CN
- School of Pharmacy, Nanjing Medical University, Nanjing, 211166, CN
- Center for Global Health, The Key Laboratory of Modern Toxicology, Ministry of Education, School of Public Health, Nanjing Medical University, Nanjing, 211166, CN School of Chemistry and Chemical Engineering, Southeast University, Nanjing, 211189, CN School of Pharmacy, Nanjing Medical University, Nanjing, 211166, CN Jiangsu Province Engineering Research Center of Antibody Drug, Key Laboratory of Antibody Technique of National Health Commission, Nanjing Medical University, Nanjing, 211166, CN

Published in Issue 13-05-2022

This work is licensed under a Creative Commons Attribution 4.0 International License.
How to Cite
Xu, Q., Zhang, L., Liu, Y., Cai, L., Zhou, L., Jiang, H., & Chen, J. (2022). Encapsulating MoS2-nanoflowers conjugated with chitosan oligosaccharide into electrospun nanofibrous scaffolds for photothermal inactivation of bacteria. Journal of Nanostructure in Chemistry, 14(2 (April 2024). https://doi.org/10.1007/s40097-022-00494-1
Abstract
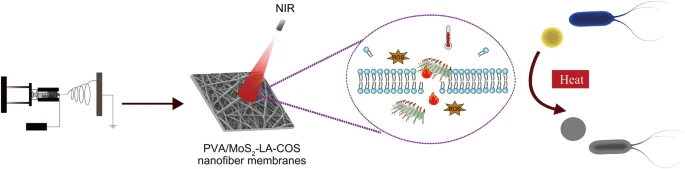
Abstract The bacterial inactivation using near-infrared (NIR) light irradiation with spatiotemporal control is advantageous to deal with the emerging microbial infection and the drug resistance in the environment and health-care facilities. Here, nanoflower-like MoS 2 prepared by electrolysis synthesis was functionalized with α-lipoic acid and chitosan oligosaccharide (MoS 2 -LA-COS) resulting in a highly biocompatible, well dispersive, and NIR photo-responsive composite. The produced nanocomposite of MoS 2 -LA-COS retained the nanoflower-like morphological feature, of which the hexagonal structure (2H phase) was similar with that of MoS 2 according to the X-ray diffraction measurement. Moreover, the produced MoS 2 -LA-COS was efficiently loaded into the electrospun nanofiber membranes (ENFs) endowing the fibrous scaffold with outstanding photothermal performance. The antibacterial studies indicated a superior bactericidal effect of the formed membranes under NIR irradiation towards the inactivation of model G − Staphylococcus aureus and G + Escherichia coli strains, which was originated from the uniform distribution of MoS 2 -LA-COS on the fibrous scaffold. The produced MoS 2 -LA-COS nanofibrous membranes of good water vapor transmission rate and improved photothermal performance possess enormous potential for the development as well as the disposal of personal protective equipment such as medical masks. Graphical abstractKeywords
- MoS2,
- Near infrared,
- Antibacterial,
- Electrospun nanofiber membranes,
- Photothermal therapy,
- Chitosan oligosaccharide
References
- Huemer et al. (2020) Antibiotic resistance and persistence-Implications for human health and treatment perspectives https://doi.org/10.15252/embr.202051034
- McEwen and Collignon (2018) Antimicrobial resistance: a one health perspective https://doi.org/10.1128/microbiolspec.ARBA-0009-2017
- Holmes et al. (2016) Understanding the mechanisms and drivers of antimicrobial resistance (pp. 176-187) https://doi.org/10.1016/S0140-6736(15)00473-0
- An et al. (2021) Plasmonic nano-antimicrobials: properties, mechanisms and applications in microbe inactivation and sensing (pp. 3374-3411) https://doi.org/10.1039/D0NR08353D
- Chen et al. (2020) Nanomaterials-based photothermal therapy and its potentials in antibacterial treatment (pp. 251-262) https://doi.org/10.1016/j.jconrel.2020.08.055
- Ashrafi et al. (2020) Antimicrobial effect of chitosan–silver–copper nanocomposite on candida albicans (pp. 87-95) https://doi.org/10.1007/s40097-020-00331-3
- González-Ballesteros et al. (2020) Synthesis of silver and gold nanoparticles by sargassum muticum biomolecules and evaluation of their antioxidant activity and antibacterial properties (pp. 317-330) https://doi.org/10.1007/s40097-020-00352-y
- Sun et al. (2019) Synergistic photodynamic and photothermal antibacterial nanocomposite membrane triggered by single NIR light source (pp. 26581-26589) https://doi.org/10.1021/acsami.9b07037
- Li et al. (2022) Injectable stretchable self-healing dual dynamic network hydrogel as adhesive anti-oxidant wound dressing for photothermal clearance of bacteria and promoting wound healing of MRSA infected motion wounds https://doi.org/10.1016/j.cej.2021.132039
- Tan et al. (2018) Rapid biofilm eradication on bone implants using red phosphorus and near-infrared light https://doi.org/10.1002/adma.201801808
- Xu et al. (2021) Nanofiber-mediated sequential photothermal antibacteria and macrophage polarization for healing MRSA-infected diabetic wounds https://doi.org/10.1186/s12951-021-01152-4
- Wei and Yu (2019) Responsive and synergistic antibacterial coatings: fighting against bacteria in a smart and effective way https://doi.org/10.1002/adhm.201801381
- Xie et al. (2018) Tuning the bandgap of photo-sensitive polydopamine/Ag(3)PO(4)/graphene oxide coating for rapid, noninvasive disinfection of implants (pp. 724-738) https://doi.org/10.1021/acscentsci.8b00177
- Aksoy et al. (2020) Photothermal antibacterial and antibiofilm activity of black phosphorus/gold nanocomposites against pathogenic bacteria (pp. 26822-26831) https://doi.org/10.1021/acsami.0c02524
- Priyadarsini et al. (2018) Graphene and graphene oxide as nanomaterials for medicine and biology application (pp. 123-137) https://doi.org/10.1007/s40097-018-0265-6
- Zhong et al. (2020) Reusable and recyclable graphene masks with outstanding superhydrophobic and photothermal performances (pp. 6213-6221) https://doi.org/10.1021/acsnano.0c02250
- Yougbaré et al. (2020) Nanomaterials for the photothermal killing of bacteria https://doi.org/10.3390/nano10061123
- Kennedy et al. (2005) An investigation of the thermal inactivation of staphylococcus aureus and the potential for increased thermotolerance as a result of chilled storage (pp. 1229-1235) https://doi.org/10.1111/j.1365-2672.2005.02697.x
- Li et al. (2019) Highly effective and noninvasive near-infrared eradication of a staphylococcus aureus biofilm on implants by a photoresponsive coating within 20 min https://doi.org/10.1002/advs.201900599
- Hao et al. (2017) In vivo long-term biodistribution, excretion, and toxicology of PEGylated transition-metal dichalcogenides MS (M = Mo, W, Ti) https://doi.org/10.1002/advs.201600160
- Oudeng et al. (2018) One-step in situ detection of miRNA-21 expression in single cancer cells based on biofunctionalized MoS2 nanosheets (pp. 350-360) https://doi.org/10.1021/acsami.7b18102
- Shi et al. (2020) Recent advances in MoS2-based photothermal therapy for cancer and infectious disease treatment (pp. 5793-5807) https://doi.org/10.1039/D0TB01018A
- Cao et al. (2017) An efficient and benign antimicrobial depot based on silver-infused MoS(2) (pp. 4651-4659) https://doi.org/10.1021/acsnano.7b00343
- Wang et al. (2015) Differences in the toxicological potential of 2D versus aggregated molybdenum disulfide in the Lung (pp. 5079-5087) https://doi.org/10.1002/smll.201500906
- Yin et al. (2016) Functionalized nano-MoS(2) with peroxidase catalytic and near-Infrared photothermal activities for safe and synergetic wound antibacterial applications (pp. 11000-11011) https://doi.org/10.1021/acsnano.6b05810
- Yuwen et al. (2021) Hyaluronidase-responsive phototheranostic nanoagents for fluorescence imaging and photothermal/photodynamic therapy of methicillin-resistant infections (pp. 4484-4495) https://doi.org/10.1039/D1BM00406A
- Ma et al. (2021) A smart nanoplatform with photothermal antibacterial capability and antioxidant activity for chronic wound healing https://doi.org/10.1002/adhm.202100033
- Chou et al. (2013) Chemically exfoliated MoS2 as near-infrared photothermal agents (pp. 4160-4164) https://doi.org/10.1002/anie.201209229
- Xu et al. (2021) A green electrolysis of silver-decorated MoS2 nanocomposite with an enhanced antibacterial effect and low cytotoxicity (pp. 3460-3469) https://doi.org/10.1039/D1NA00100K
- Yang et al. (2020) Electrochemical generation of liquid and solid sulfur on two-dimensional layered materials with distinct areal capacities (pp. 231-237) https://doi.org/10.1038/s41565-019-0624-6
- Xu et al. (2020) Electrochemical formation of distinct nanostructured MoS2 with altered antibacterial activity https://doi.org/10.1016/j.matlet.2020.127809
- Croisier et al. (2015) Chitosan-coated electrospun nanofibers with antibacterial activity (pp. 3508-3517) https://doi.org/10.1039/C5TB00158G
- Kurtz and Schiffman (2018) Current and emerging approaches to engineer antibacterial and antifouling electrospun nanofibers https://doi.org/10.3390/ma11071059
- He et al. (2020) Anti-oxidant electroactive and antibacterial nanofibrous wound dressings based on poly(ε-caprolactone)/quaternized chitosan-graft-polyaniline for full-thickness skin wound healing https://doi.org/10.1016/j.cej.2019.123464
- Zhao et al. (2021) Injectable dry cryogels with excellent blood-sucking expansion and blood clotting to cease hemorrhage for lethal deep-wounds, coagulopathy and tissue regeneration https://doi.org/10.1016/j.cej.2020.126329
- Sepahi et al. (2020) Introducing electrospun polylactic acid incorporating etched halloysite nanotubes as a new nanofibrous web for controlled release of amoxicillin (pp. 245-258) https://doi.org/10.1007/s40097-020-00362-w
- Wang et al. (2020) Highly efficient transparent air filter prepared by collecting-electrode-free bipolar electrospinning apparatus https://doi.org/10.1016/j.jhazmat.2019.121535
- Buivydiene et al. (2019) Formation and characterisation of air filter material printed by melt electrospinning (pp. 48-63) https://doi.org/10.1016/j.jaerosci.2019.03.003
- Zhu et al. (2019) Bio-based and photocrosslinked electrospun antibacterial nanofibrous membranes for air filtration (pp. 55-62) https://doi.org/10.1016/j.carbpol.2018.09.075
- Kim et al. (2004) Incorporation and controlled release of a hydrophilic antibiotic using poly(lactide-co-glycolide)-based electrospun nanofibrous scaffolds (pp. 47-56) https://doi.org/10.1016/j.jconrel.2004.04.009
- Gizaw et al. (2018) Electrospun fibers as a dressing material for drug and biological agent delivery in wound healing applications
- Zhang et al. (2019) Silver-infused porphyrinic metal-organic framework: surface-adaptive, on-demand nanoplatform for synergistic bacteria killing and wound disinfection https://doi.org/10.1002/adfm.201808594
- Stanislav Presolski (2016) Covalent functionalization of MoS2 (pp. 140-145) https://doi.org/10.1016/j.mattod.2015.08.019
- Liu et al. (2014) Drug delivery with PEGylated MoS2 nano-sheets for combined photothermal and chemotherapy of cancer (pp. 3433-3440) https://doi.org/10.1002/adma.201305256
- Zhang et al. (2021) Alginate-chitosan oligosaccharide-ZnO composite hydrogel for accelerating wound healing https://doi.org/10.1016/j.carbpol.2021.118100
- Yue et al. (2020) Cinnamyl alcohol modified chitosan oligosaccharide for enhancing antimicrobial activity https://doi.org/10.1016/j.foodchem.2019.125513
- Yan et al. (2015) Polydopamine-coated electrospun poly(vinyl alcohol)/poly(acrylic acid) membranes as efficient dye adsorbent with good recyclability (pp. 730-739) https://doi.org/10.1016/j.jhazmat.2014.10.040
- Liu et al. (2018) Electrochemical synthesis and tribological properties of flower-like and sheet-like MoS2 in LiCl KCl (NH4)6Mo7O24KSCN melt (pp. 252-260) https://doi.org/10.1016/j.electacta.2018.03.015
- Xu et al. (2017) Integration of IR-808 sensitized upconversion nanostructure and MoS2 nanosheet for 808 nm NIR light triggered phototherapy and bioimaging https://doi.org/10.1002/smll.201701841
- Franco et al. (2012) On stabilization of PVPA/PVA electrospun nanofiber membrane and its effect on material properties and biocompatibility https://doi.org/10.1155/2012/393042
- Liu et al. (2020) Enhanced antimicrobial activity and pH-responsive sustained release of chitosan/poly (vinyl alcohol)/graphene oxide nanofibrous membrane loading with allicin (pp. 1405-1413) https://doi.org/10.1016/j.ijbiomac.2020.08.051
- Chang et al. (2016) Targeted synthesis of 2H- and 1T-phase MoS2 monolayers for catalytic hydrogen evolution (pp. 10033-10041) https://doi.org/10.1002/adma.201603765
- Zhang et al. (2016) The surface grafting of graphene oxide with poly(ethylene glycol) as a reinforcement for poly(lactic acid) nanocomposite scaffolds for potential tissue engineering applications (pp. 403-413) https://doi.org/10.1016/j.jmbbm.2015.08.043
- Wang et al. (2020) Boron-modified defect-rich molybdenum disulfide nanosheets: reducing nonspecific adsorption and promoting a high capacity for isolation of immunoglobulin G (pp. 43273-43280) https://doi.org/10.1021/acsami.0c12171
- Lawrie et al. (2007) Interactions between alginate and chitosan biopolymers characterized using FTIR and XPS (pp. 2533-2541) https://doi.org/10.1021/bm070014y
- Liu et al. (2014) Solution blowing of chitosan/PVA hydrogel nanofiber mats (pp. 1116-1121) https://doi.org/10.1016/j.carbpol.2013.10.056
- Teng et al. (2016) MoS2 nanosheets vertically grown on graphene sheets for lithium-ion battery anodes (pp. 8526-8535) https://doi.org/10.1021/acsnano.6b03683
- Merki et al. (2011) Amorphous molybdenum sulfide films as catalysts for electrochemical hydrogen production in water (pp. 1262-1267) https://doi.org/10.1039/C1SC00117E
- Tang et al. (2018) Acidity/reducibility dual-responsive hollow mesoporous organosilica nanoplatforms for tumor-specific self-assembly and synergistic therapy (pp. 12269-12283) https://doi.org/10.1021/acsnano.8b06058
- Yin et al. (2014) Multifunctional upconverting nanoparticles for near-infrared triggered and synergistic antibacterial resistance therapy (pp. 10488-10490) https://doi.org/10.1039/C4CC04584J
- Yong et al. (2017) Polyoxometalate-based radiosensitization platform for treating hypoxic tumors by attenuating radioresistance and enhancing radiation response (pp. 7164-7176) https://doi.org/10.1021/acsnano.7b03037
- Bhardwaj and Kundu (2010) Electrospinning: a fascinating fiber fabrication technique (pp. 325-347) https://doi.org/10.1016/j.biotechadv.2010.01.004
- Cheng, F., Gao, J., Wang, L., Hu, X.: Composite chitosan/poly (ethylene oxide) electrospun nanofibrous mats as novel wound dressing matrixes for the controlled release of drugs. J. Appl. Polym. Sci.
- 132
- (2015).
- https://doi.org/10.1002/APP.42060
- Jannesari et al. (2011) Composite poly(vinyl alcohol)/poly(vinyl acetate) electrospun nanofibrous mats as a novel wound dressing matrix for controlled release of drugs (pp. 993-1003)
- Li et al. (2021) Photocatalytic rejuvenation enabled self-sanitizing, reusable, and biodegradable masks against COVID-19 (pp. 11992-12005) https://doi.org/10.1021/acsnano.1c03249
- Huang et al. (2020) Self-Reporting and photothermally enhanced rapid bacterial killing on a laser-induced graphene mask (pp. 12045-12053) https://doi.org/10.1021/acsnano.0c05330
- Lin et al. (2020) Superhydrophobic, photo-sterilize, and reusable mask based on graphene nanosheet-embedded carbon (GNEC) film (pp. 1110-1115) https://doi.org/10.1007/s12274-020-3158-1

 10.1007/s40097-022-00494-1
10.1007/s40097-022-00494-1