Graphical Abstract

In this work, amino-functionalized SBA-15 (SBA-Pr-NH 2 ) has been used as a basic nano-catalyst in the one-pot synthesis of 2-aryloxazolo[4,5- b ]pyridines by the condensation reaction of 2-amino-3-hydroxypyridine and benzoyl chlorides under microwave irradiation in solvent free conditions. Among the advantages of this work are short reaction times, high yields and simple work-up. SBA-Pr-NH 2 as an efficient heterogeneous nanoporous solid basic catalyst which was prepared by silica functionalization with 3-aminopropyltriethoxysilane, can be easily removed from the reaction mixture by simple filtration, and also recovered and reused without noticeable loss of reactivity. 2-Aryloxazolo[4,5- b ]pyridines have different pharmacological activities (anti-inflammatory, anti-pyretic, analgesic, antifungal). In the current method for the synthesis of 2-aryloxazolo[4,5- b ]pyridines, no hazardous organic solvent was used and simplicity of the procedure provides significant improvements over many of other existing methods. Furthermore, the SBA-15-Pr-NH 2 with pore size of 6 nm can act as nano-reactor.

Oxazolopyridines have attracted much interest in diverse areas of chemistry. These heterocycles have shown different pharmacological activities such as anti-inflammatory, anti-pyretic, analgesic [ 1 – 3 ], and antifungal properties [ 4 ]. They can also act as inhibitor of fatty acid amide hydrolaze (FAAH) [ 5 , 6 ], inhibitors of monoterpenoidindole alkaloid production in Catharanthus roseus cells [ 7 ], inhibitors of acetylcholinesterase/butyrylcholinesterase [ 8 ], and SIRT1 activators [ 9 ].
The oxazolopyridine structure can be found in some compounds such as
Ι
and
П
that showed moderate activation of SIRT1 and also in 6-benzoylbenzoxazolinone
Ш
(Fig.
1
) which has high analgesic activity comparable to indomethacin.
Structure of some compounds with oxazolopyridine scaffoldFig. 1
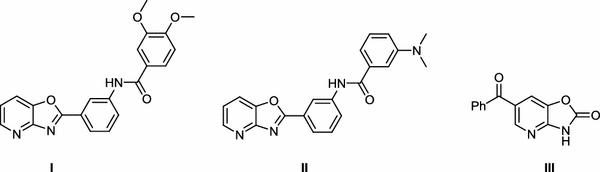
These compounds have been investigated in numerous studies for their applications as pesticides [ 7 ] and dyes [ 1 , 10 ]. Fluorescence emissions in the visible region of the spectrum are characterization of many oxazolopyridine derivatives. This property makes them good candidates for construction of LED devices or nonlinear optic systems [ 1 ].
On the other hand, there are few routes to oxazolopyridines which are very similar to benzoxazoles [ 11 ]. A number of methods have been reported for the synthesis of these heterocycles which include condensation of carboxylic acids [ 1 , 12 , 13 ], orthoesters [ 14 ], acid chlorides and nitriles [ 8 ], with o -substituted amino aromatics. Other methods include Pd-catalyzed C-2 arylation of oxazolo[4,5- b ]pyridine [ 5 ], reaction of o -aminopyridinols with aldehydes under oxidative conditions and cyclization of an o -halogeno amidopyridine at very high temperature [ 11 ].
Recently, there has been increased interest on the application of functionalized mesoporous silica materials in different fields such as catalysis [ 15 ], drug delivery [ 16 ], and photocatalysts [ 17 ]. Post-synthetic functionalization of the surface of SBA-15 by 3-aminopropyltriethoxysilane (APTES), resulted in the formation of SBA-Pr-NH 2 as amino-functionalized SBA-15 [ 18 ] that has been used as catalyst in a variety of reactions [ 19 , 20 ].
Herein, as part of our ongoing interest in the application of heterogeneous solid catalysts in organic synthesis [ 21 – 23 ], we want to report the development of a one-pot and green microwave-assisted protocol in the presence of SBA-Pr-NH 2 for the synthesis of 2-aryloxazolo[4,5- b ]pyridine derivatives.
In this investigation, the synthesis of N
1
-(3-hydroxy-2-pyridyl)benzamide derivatives
3
and 2-aryloxazolo-[4,5-
b
]pyridines
5
from the condensation of 2-amino-3-hydroxypyridine
1
and benzoyl chlorides
2
in the presence of SBA-Pr-NH
2
as a heterogeneous and reusable nano-catalyst and under microwave irradiation in solvent free condition was studied (Scheme
1
).
Reaction of 2-amino-3 hydroxypyridine 1 and benzoyl chlorides 2Scheme 1
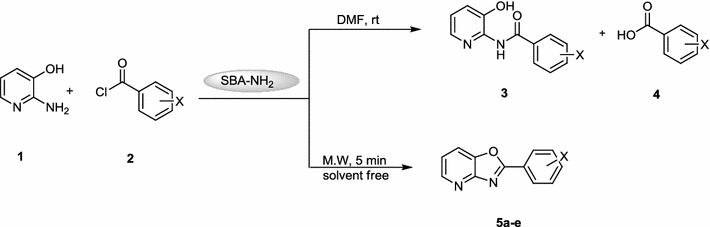
At first, the reaction of 2-amino-3-hydroxy pyridine 1 and 4-chloro benzoyl chloride 2 was examined as a model experiment in dimethylformamide (DMF) as solvent at room temperature in the presence of SBA-Pr-NH 2 . In this condition, the hydrolysis of benzoyl chloride to corresponding benzoic acid was occurred and only 2-methyl benzoyl chloride resulted in the formation of the acylated product (N 1 -(3-hydroxy-2-pyridyl)benzamide) 3 as intermediate.
To investigate the effect of temperature, the progress of reaction mixture was also studied under reflux condition. It was found that the reaction time was too long and two products: the acylated product
3
and oxazolo-[4,5-
b
]pyridine derivatives
5
were obtained. It could be concluded that under reflux conditions, compound
3
was converted to aryloxazolopyridine derivatives
5
in moderated yields. For more investigations, a broad range of structurally diverse benzoyl chlorides
2
was condensed with 2-amino-3-hydroxy pyridine
1
under room temperature (Method A) and also at conventional heating (Method B). GC-Mass analysis of condensation products at mentioned conditions is summarized in Tables
1
, and
2
.
Ratio of products in the presence of SBA-Pr-NH
2
(Method A) Entry X Method Aa Product’s ratiob 3 (%) 4 (%) 5 (%) 1 2-Cl – 100 – 2 3-Cl 60 37 3 3 4-Cl – 100 – 4 2,4-(Cl)2 – 100 – 5 3-NO2 – – 50 6 2-Me 100 – – Ratio of products in the presence of SBA-Pr-NH
2
(Method B) Entry X Method Ba Product’s ratiob 3 (%) 4 (%) 5 (%) 1 2-Cl 3 – 97 2 3-Cl – 35 65 3 4-Cl – 65 35 4 3-NO2 44 – 56 5 2-Me 44 36 20Table 1
Table 2
At the next step, we used microwave irradiation instead of conventional heating. The reaction of this one-pot synthesis was generally fast and clean and went to completion in microwave oven for about 5 min at the maximum power level. The insolubility of SBA-Pr-NH
2
in different organic solvents led to very easy work up of catalyst by simple filtration. After cooling of the filtrate and evaporation of solvent, the pure crystals of products were obtained. In use of microwave irradiation, good to excellent yields were obtained without formation of any side products such as benzoic acids which are normally observed at room temperature or reflux conditions. The generality of this procedure was evaluated by the reactions of various benzoyl chlorides with 2-amino-3-hydroxypyridine. As shown in Table
3
, in the most cases, 2-amino-3-hydroxypyridine completely reacted with a wide range of substituted benzoyl chlorides and afforded the corresponding 2-aryloxazolo[4,5-
b
]pyridines in good to excellent yields. The benzoyls with electron-withdrawing groups (Table
3
, entries 1–4) which were predicted to be more reactive toward compound
1
led to oxazolo-[4,5-
b
]pyridine derivatives
5
in higher yields. On the other hand, electron-donating substituent on the aromatic ring of benzoyl chloride decreased the yield of reaction (Table
3
, entry 5).
Synthesis of 2-aryloxazolo[4,5-
b
]pyridines in microwave condition Entry Product Yield (%) m.p (°C) m.p (References) 1 100 128–130 142–143 [ 2 86 158–159 160–162 [ 3 54 217–218 New 4 78 188–190 199–201 [ 5 46 98–99 64–66 [Table 3
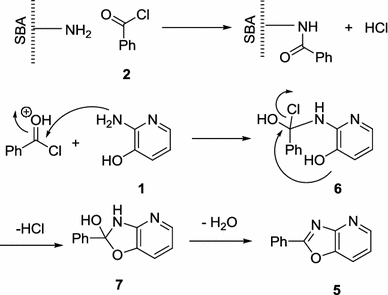
The suggested mechanism for the synthesis of 2-aryloxazolo[4,5-
b
]pyridines
5
is shown in Scheme
2
. The amines of SBA-Pr-NH
2
are so active that react with acyl chlorides and form HCl. Then, nucleophilic addition of 2-amino-3-hydroxypyridine
1
to protonated benzoyl chloride
2
produces compound
6
which subsequent elimination of HCl, ring closure, and dehydration leads to the formation of final product 2-aryloxazolo[4,5-
b
]pyridines
5
.
The proposed mechanism for the synthesis of 2-aryloxazolo[4,5-
b
]pyridines 5Scheme 2
The synthesis of oxazolopyridine derivatives
5
has been studied with several catalysts and solvents in literature as shown in Table
4
. In contrast with other existing methods, the present methodology offers several advantages such as excellent yields, a simple procedure, easy synthesis, simple work-up and greener conditions. The product structures were confirmed by IR,
1
H NMR,
13
C NMR and GC-Mass data. Melting points were compared with reported values in literature.
Efficiency comparison of various catalysts in synthesis of 2-aryloxazolo[4,5-
b
]pyridines Entry Precursor Catalyst Solvent Condition Time Yield (%) References 1 Carboxylic acids – – MW 2–12 min 18–85 [ 2 Aryl halide Pd(OAc)2/PPh3/Cs2CO3 Acetone 30 °C 24 h 33–74 [ 3 Benzoyl chloride Trimethylsilyl polyphosphate (cyclization agent) 1,2-Dichlorobenzene Reflux 15 h – [ 4 Benzoic anhydride – – Reflux Several min ~34 [ 5 Benzoic acid Polyphosphoric acid – 130–230 °C 10 min ~62 [ 6 Orthoester – 140–180 °C – 30–60 [ 7 Orthoester ZrOCl2·8H2O – rt or 85 °C 12 min 88–89 [ 8 Benzoic acid Polyphosphoric acid (85 % phosphorus pentoxide) – 160 6 h – [ 9 Benzoic acid PPh3, Et3N (excess) CCl4 Reflux – 72–99 [ 10 Benzoyl chloride SBA-NH2 – MW 5 min 46–100 This workTable 4
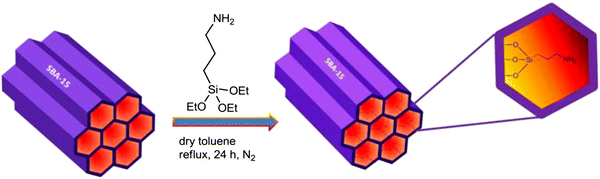
The amino-functionalized SBA-15 was typically synthesized through post-grafting. A schematic illustration of the preparation of SBA-Pr-NH
2
is shown in Fig.
2
. The calcined SBA-15 silica was functionalized with 3-aminopropyltriethoxysilane (APTES, 99 %) to provide SBA-Pr-NH
2
as solid basic nano-catalyst.
Schematic representation of SBA-Pr-NH
2Fig. 2
Figure
3
illustrates the Transmission Electron Microscopy (TEM) image of SBA-NH
2
. The TEM image showed the parallel channels, which resembled the configuration of the pores in SBA-15.
TEM image of SBA-Pr-NH
2Fig. 3
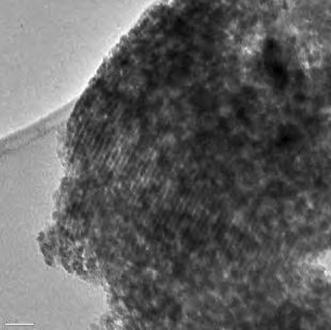
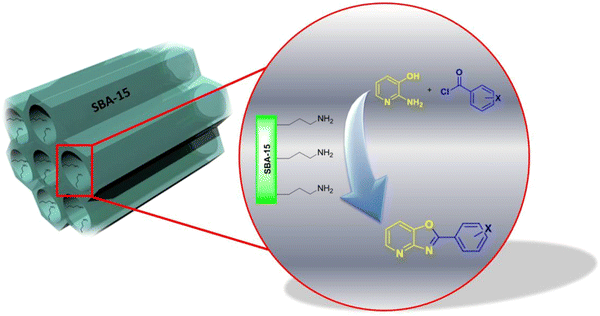
Nano pore size about 6 nm of SBA-Pr-NH
2
could act as a nano-reactor which catalyzed the synthesis of 2-aryloxazolo[4,5-
b
]pyridine derivatives. A schematic illustration for this activity is shown in Fig.
4
.
SBA-Pr-NH
2
acts as a nano-reactorFig. 4
In summary, we have described the use of microwave in the synthesis of 2-aryloxazolo[4,5- b ]pyridine derivatives in the presence of SBA-Pr-NH 2 . The use of microwave irradiation under solvent free conditions makes this protocol practical and environmentally friendly. The operational and experimental simplicity, readily available materials, simple work-up procedure, very short reaction times, and good yields of products are other advantages of this protocol which make it a facile method for the synthesis of these compounds.
The chemicals employed in this work were obtained from Merck Company and were used with no purification. Pluronic P123 (EO 20 PO 70 EO 20 , M ac = 5,800) block copolymer non-ionic surfactant was purchased from Sigma Aldrich. Tetraethyl orthosilicate (TEOS, 98 %), 3-aminopropyltriethoxysilane (APTES, 99 %), hydrochloric acid (HCl, 37 %), and toluene were purchased from Merck (Germany). GC-Mass analysis (Gas chromatography–mass spectrometry) was performed on a GC-Mass model: 5973 network mass selective detector, GC 6890 Agilent. IR spectra were obtained with a Bruker 500 scientific spectrometer. The 1 H NMR and 13 C NMR were run on a Bruker DPX, at 250 and 62.5 MHz using TMS as the internal standard. Melting points were measured using the capillary tube method with an electrothermal 9200 apparatus. Microwave-assisted reactions were carried out using a MicroSYNTH Microwave Labstation. Transmission Electron Microscopy (TEM) analysis was performed on a Tecnai G 2 F30 at 300 kV.
The nanoporous compound SBA-15 was synthesized and functionalized according to our previous report [ 19 , 28 ] and the modified SBA-Pr-NH 2 was used as a nanoporous solid basic catalyst in the following reaction.
The SBA-Pr-NH 2 (0.01 g) was activated in vacuum at 100 °C and then after cooling to room temperature, 2-amino-3-hydroxypyridine (3 mmol, 0.33 g) and appropriate benzoyl chloride (3 mmol) were mixed in a glass tube and irradiated in the microwave oven for about 5 min at maximum microwave power level (700 W). After completion of reaction which was indicated by TLC ( n -hexane/EtOAc, 1:1), hot DMF was added to dissolve the crude products. Then the catalyst was removed from reaction mixture by simple filtration, and water was added to mixture to obtain the solid products.
1 H NMR (250 MHz, DMSO- d 6 , δ, ppm): 6.95–7.11 (m, 1H), 7.54 (m, 1H), 7.72 (m, 1H), 7.95 (m, 1H), 8.25 (m, 1H), 8.61 (m, 1H). 13 C NMR (62.5 MHz, DMSO- d 6 , δ, ppm): 120.0, 122.0, 124.3, 128.0, 131.5, 133.8, 134.1, 138.0, 143.0, 147.5, 155.3, 162.4. IR (KBr, cm −1 ): υ max = 3,068, 1,602, 1,260, 786. MS ( m / z (%)): 264 (100), 171 (5), 136 (5), 118 (5), 93 (53), 65 (13).
We gratefully acknowledge for financial support from the Research Council of Alzahra University and University of Tehran.
The authors declare that they have no competing interests.
MSHN carried out the synthesis, purification and characterization of the compounds and has been supervised by GMZ who supervised the project and also she is the corresponding author. AB carried out the catalyst design and prepared the nano catalyst. NL helped to draft the manuscript and rechecked the manuscript. All authors read and approved the final manuscript.